Food supplements are becoming increasingly popular among Indian customers. This is due to the consumer’s understanding of the significance of consuming healthy meals & ever-increasing concern for the environment. Furthermore, customer per capita disposable earning has also increased significantly, enabling them to make ample spending on health supplements. The inclination of Indian consumers toward food supplements is strengthening every passing day, which forcing local manufacturers to crank up the production rate accordingly. Food supplements are created through a sophisticated process and usually constitute a long list of substances in a viable proportion. Since the effect of these products varies from person to person, their manufacturers have to comply with various standards and norms to intact their potency and legitimacy. The presence of inferior and duplicate products has forced the government to tighten the prevailing laws. FSSAI, the leading food regulator of India, has already taken countless measures to keep the sub-par product out of the market. In this blog, we will unfold FSSAI Guidelines for Food Supplements in detail.
An Outlook FSSAI Guidelines for Food Supplements Business
In the past few years, the FSSAI Guidelines for food supplements have undergone numerous changes owing to the increasing health issues and dominance of identical products in the market. Food supplements are usually available to the customers in the form of capsules, tablets, syrups, and more.
These products should meet the quality norms as cited in the Indian Pharmacopoeia, British Pharmacopoeia, or United States[1] Pharmacopoeia. In India, as Food business operators, you may apply for Basic FSSAI registration if your annual turnover is below under Rs 12 Lac. Similarly, you can also opt for state and central licenses if your turnover range is in accordance with the following threshold limits outlined by the FSSAI.
Read our article:Renew FSSAI License – When and How to Do it!
Type of food license Annual Turnover Threshold
For State License Rs 12 lacs-20 crores
For Central License More than Rs 20 crores
The formulation of the food supplements must be based on fundamental nutrition & medicinal principles. Furthermore, these products must be supported by properly validated scientific data.
A simple blend of vitamins & minerals formulated in the form of tablets, capsules, or syrup cannot be deemed as a food article a legit food format is followed by adding specific vitamins & minerals. These foods articles should be free from hormones, steroids, or any psychotropic ingredients.
The type of nutrients present in the food supplements should meet the threshold allowance specified by the Indian Council of Medical Research.
The applicability of Codex Alimentarius Commission* standards will not come to effect if the specs pertaining to these standards are not clear.
FBOs are eligible to use approved additives & colours in view of Schedule VF regulations. Moreover, Food Safety and Standards (Food Product Standards and Food Additives) Regulations, 2011, permits manufacture to add natural or synthetic flavours in a viable proposition.
Labelling Prerequisites to be Followed Food Supplement Business
There are some norms pertaining to the labelling on the food items as per the Food Safety & Standards (Packaging & Labelling) Regulations, 2011 & other norms mention on each food type. The label must reflect the given information
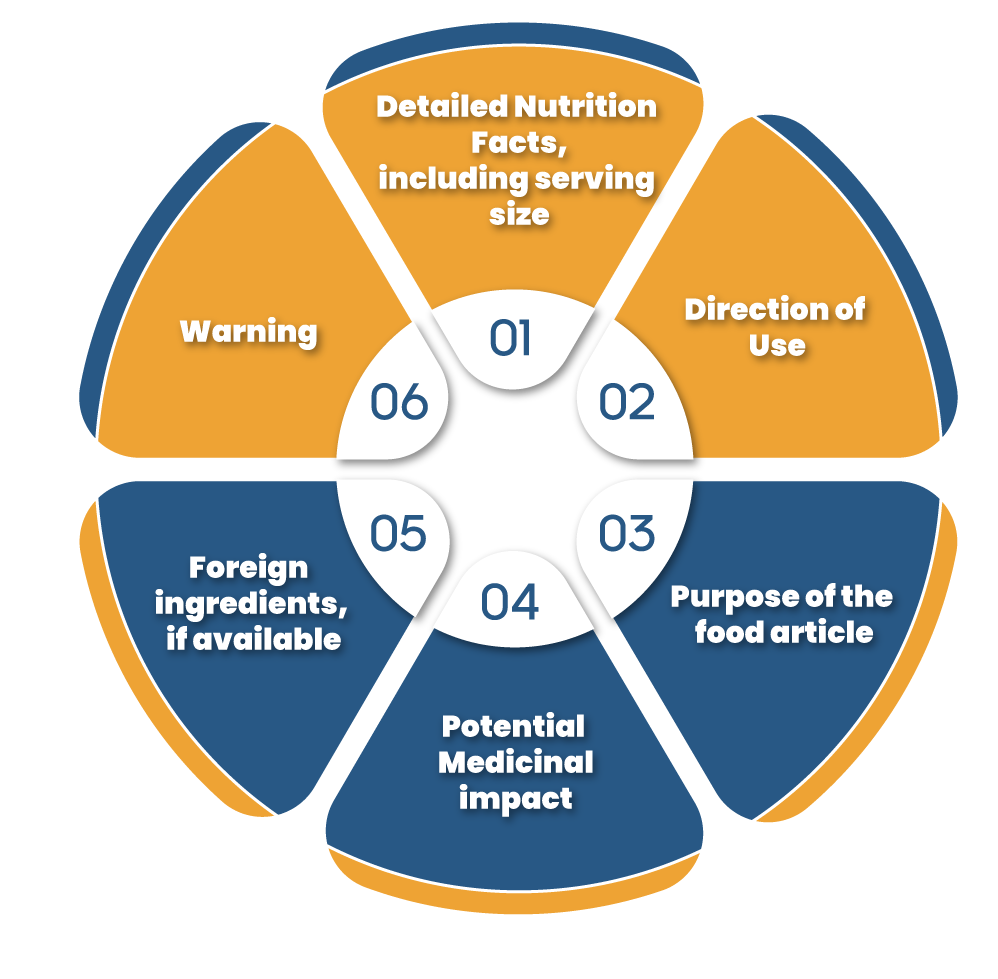

The labelling of food supplements should manifest the following fact when it comes to nutritional information.
- energy value in kcal
- the amounts of carbs, protein, and fat in gram (g) or ml;
- the amount related to their nutrients for which health claim is made
An unmodified food item that is free from any foreign substance cannot be deemed as “special dietary”,
Such food items accompany a statement on its label reflecting the type of food. Here X reflects the mandatory distinctive traits as validated by scientific data that is generally accepted. The FBOs should abide by such conditions and keep the details as authentic as possible.
FSSAI Guidelines for Food Supplements Related to Additives & Product Ingredients
Definitive and subtle schedules divide norms with detailed norms where information regarding the use of vitamins, minerals, amino acids, botanical ingredients, prebiotics, nutraceutical ingredients, food additives is shared.
There exist a listicle of 400 ingredients related to botanically origin plant that are accessible to FBOs. These ingredients are covered in one of the schedules, and FBOs can use them as food ingredients as per the regulations.
According to the regulatory provisions, the ingredients available under the schedules from Ist to VIIth are available to FBOs. Likewise, in light of these regulations, the additives can also be utilized as applied to the classification cited in Schedule VA to VF.
Provision relating to Health or Nutritional Claims made by FBOs
Nutritional claims can be made by manufacturers for ingredients that have been cited in Schedules I-VI. These claims can be shared based on:
- Health-based benefits
- Nutritional ingredients
The following can be taken into account when it comes to sharing the health claims
- Improved Function
- Ingredients or nutritional function
- Disease risk reduction
- Anti-aging
- Immunity or increased resistance
- Health maintenance claims
FBOs need to verify health claims via accurate and adequate documentation. Further, human studies can be taken into account in this context.
In case of the declaration of other claims, it is vital to get them validated by the concerned authority. Implied claims related to the cure of certain diseases or making efficacy claims by name, symbols or vignettes, are strictly prohibited by FSSAI.
Limitations on Selling of Certain Food Supplements in India
The FSSAI also imposes a limitation on the trading of certain food items that are available in the market:
- Food items that do not possess a clear distinction between food items available for human consumption
- Food items that fail to abide by the claimed nutritional purpose
- Food items that is detrimental to human life
Conclusion
The way people are prioritizing their health at present has raised the requirement for tightening the norms for food supplement in India. People are expecting strong norms from regulators like FSSAI to keep the non-legitimate product out of the market. Since these products churn out good profit margins, it gave leverage to the offender to produce duplicate products. It is needless to mention that such products could cause complete wreak havoc on one’s health. FSSAI is taking such adversities very seriously and endeavours to patch up every loophole that may hinder end-users’ interest. Existing FSSAI regulations for food supplements seem to be spot on as far as the customer’s health and safety are concerned.
Read our article:Everything you need to know about FSSAI License Renewal & Validity











