Overview of Manufacturing Drug license
Manufacturing Drug License is the license issued by the competent authority under the Drugs and Cosmetic Act, 1940 to start a business manufacturing drugs/medicines or cosmetics. The State Licensing Authority is responsible for granting the license such as:
The government has strict rules for granting the Manufacturing and sales of drugs under the Drugs and Cosmetics Act, 1940. The other functions carried out by the authorities are to perform a regular inspection of the premises and drugs manufacturing unit and to stop malpractices by implementing the Food and Drugs Act. All types of drugs include Ayurvedic, Allopathic, Homeopathic and Cosmetics. The drug license helps the authority monitor and maintain the quality of drugs sold in India.
Application to obtain a manufacturing drug license is made to the state drug licensing authority, CDSCO Zonal/Sub-zonal office or the CDSCO, i.e., the Drug Controller General of India.
Pre-requisites for Obtaining a Manufacturing Drug License
Before obtaining the Manufacturing Drug License, an applicant must fulfil the following Pre-requisites:
Benefits of Obtaining the Manufacturing Drug License
The Benefits of Obtaining the Manufacturing Drug License are the following:
- Legality
A person must file a Manufacturing drug license application as it provides legality to the business. It is also illegal to engage in the Manufacturing or sale of drugs in India without obtaining a proper license.
- Proper Monitoring and Regulation by The Authority
Manufacturing drug license helps the authority to monitor and regulate the sale of medicines in India, which ultimately enhances business.
- Proof of Authenticity
A Manufacturing Drug license plays a vital role, as it works as a proof of authenticity and builds an image of the company.
- Confidence In the Consumers
A drug license certificate proves to the consumers that the manufacturer is following strict quality measures while manufacturing drugs. Also, a valid license proves to the consumers that the drugs are safe and constitute no health hazards.
Book a Free Consultation
Get response within 24 hours
Documents required for Manufacturing Drug License
The followings are the required Documents for obtaining the Manufacturing Drug License:
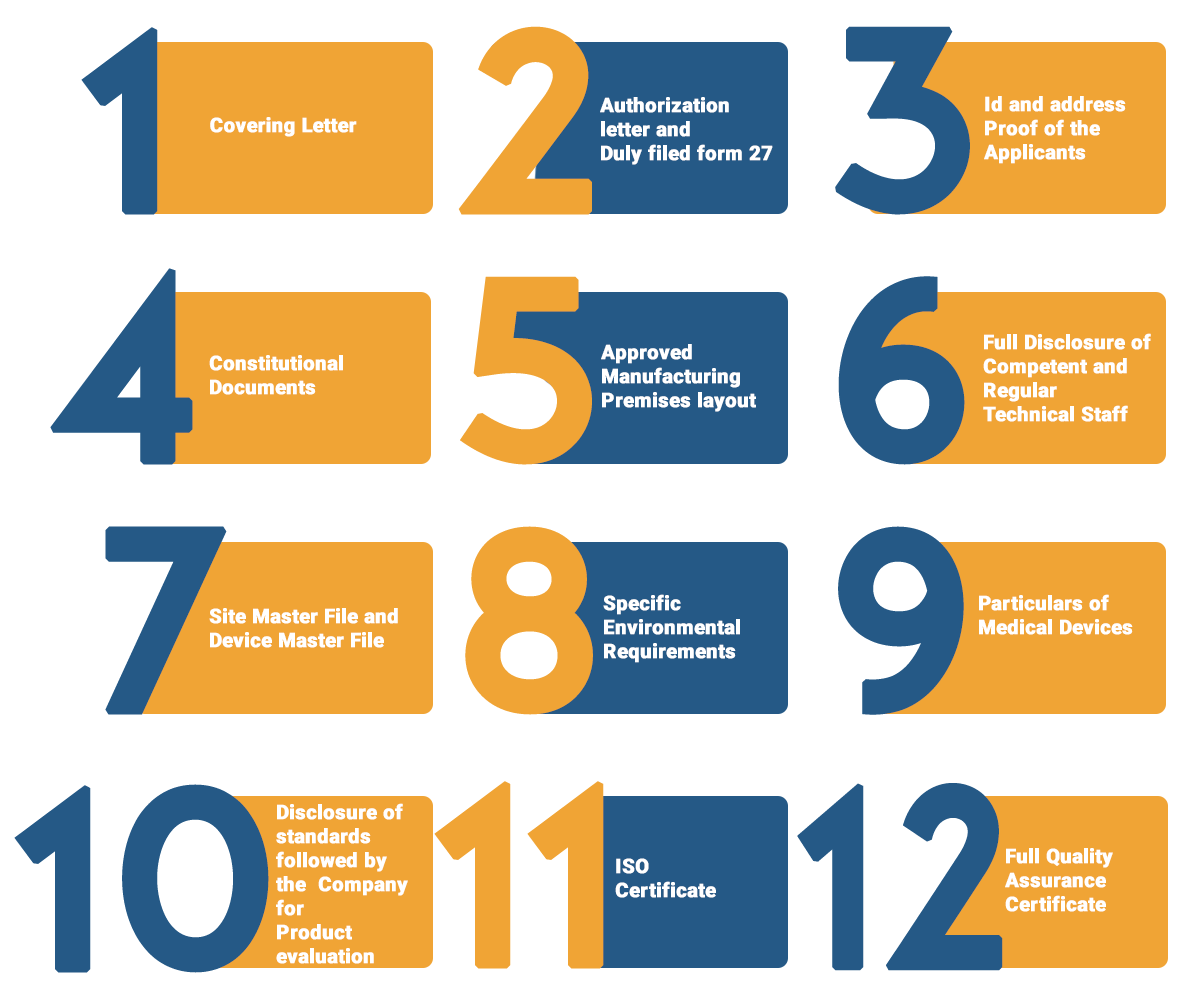
Apart from the Documents mentioned above, additional Documents are required for obtaining the Manufacturing Drug license:
Proof Of Possession
Proof Of Ownership
Procedure for Filing the Manufacturing Drug License
The procedure to follow while applying for the Manufacturing Drug License is mentioned below:
- Preparation of Documents
The very initial step is preparing the requisite Documents. The applicant must obtain all the Documents and get them attested by the authorities.
- Filing the application
The applicant has to fill online application form by visiting the official website of their respective state licensing authority. After filing the form and u[ploading all the required Documents, the applicant will have to pay the required government fee for the same.
However, in certain states, applicants will have to take a print of the application submitted online along with the Documents and send it to the District Licensing Office.
- Verification By the Authority
A drug inspector will visit the premises to verify the submitted Documents and to ensure that the premises are suitable for manufacturing drugs. After verification, the Drug inspector might call the applicant for an interview.
- Issue Of Drug License
Once verification is complete, and the authority is satisfied, the Controller of Drugs will issue a manufacturing drug license for the manufacturer. Once a registration is done successfully, the applicant will receive a unique Registration Number which they can use for further processing and reference.
CorpBiz Assistance in obtaining Manufacturing Drug License
Frequently Asked Questions
- Manufacturing License
- Sale License
- Wholesale Drug License
- Retail Drug License
- Restricted Drug License
- Loan License
- Import License
- Multi-drug License
A Restricted Drug license is a license granted to those dealers who do not hold the services of a qualified person and only deal with such classes of drugs whose sales can be affected without a qualified person who do not have fixed place of business.
- Manufacturing Drug License is compulsory for the drugs, medicine, or cosmetics business.
- It shall be permitted for a commercial premise
- It shall comply with the requirement and conditions imposed by law and authorities and shall be displayed all the time in the place of business.
The Drugs Controller General (India) grants the registration certificate vide Gazette notification G.S.R 426(E) under the provisions of Drugs and Cosmetics Act, 1940 and Rules made thereunder.
Form 11 under the Drugs and Cosmetics Act is issued for the import of small quantities of drugs for examination testing or analysis.
- Any individual,
- Company,
- partnership firm,
- Limited Liability Partnership,
- One Person Company can apply for a Drug license.
A holder can submit the complete application with the following enclosures in the office of the Drug Licensing Authority of your district within 15 days of online registration for renewal in a duly filled Form 19/19A/19AA/19C.
Hand sanitizers are licensed under Drugs and Cosmetics Rules, 1945. The standards of such products must be prescribed in the Second Schedule of Drugs and Cosmetics Act & rules made there under. Further, on March 13, 2020, the central government had notified masks (2ply & 3ply surgical masks, N95 mask) & hand sanitizers under Essential Commodities Act, 1955 in order to regulate their production, quality, distribution and logistics.
A subsequent Drug license implies a medication registered by the Central Licensing Authority for certain cases and preferred to be advertised with changed or new cases including sign, course of organization, measurements, and dose structure.
No manufacturing, processing, packing, or holding of prescription or nonprescription drugs shall be conducted in any personal accommodation.
 See How It Works in 1 min video
See How It Works in 1 min video

























