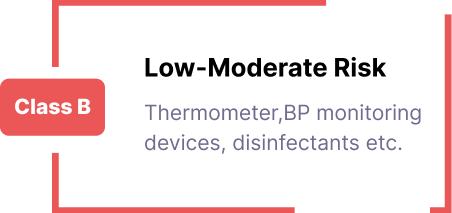
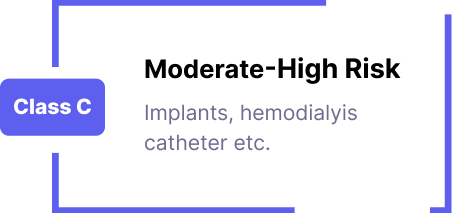
CDSCO Registration for Medical Devices/IVDs/Cosmetics
Corpbiz guarantees to provide you with 100% assistance in CDSCO Registration of Medical Devices, IVDs, or Cosmetics in India by maintaining transparency. Also, we have more than 250+ CDSCO experts all over India who have done many CDSCO Registrations in all over India.