The FSSAI has come up with the new regulating law which is named as Food Safety and Standards (Food for Infant Nutrition) Regulations, 2019. This particular Act is regarding infant nutrition and health for the babies not above the age of 12 months.
Although this Food for Infant Nutrition Regulation existed as part of the Food Safety & Standards (Food Products Standards & Food Additives) Regulations, 2011[1]. However, now the FSSAI has framed a separate regulation which has been implemented. This new regulation is more comprehensive.
What has been Mention in Foods for Infant Nutrition Regulation?
Initially, the FSSAI has invited comments, objections, and suggestions from stakeholders before formally implanting the final act before 14 June 2019. Now the act has came into force in 2019 with the name Food Safety and Standards (Foods for Infant Nutrition) Regulations, 2019.
The regulation includes the standards for Infant formula for special medical purpose especially food for infants who are inborn with the (IEM) Errors of Metabolism. The standards have been mentioned in regard to premature infant such as milk substitutes, Lactose and sucrose free infant milk substitutes which is made under infant formula for the special medical purposes. In the Act there has been mentioned regarding the standards for food for infant nutrition based on traditional food ingredients.
Some Important Terms in Foods for Infant Nutrition Regulation 2019
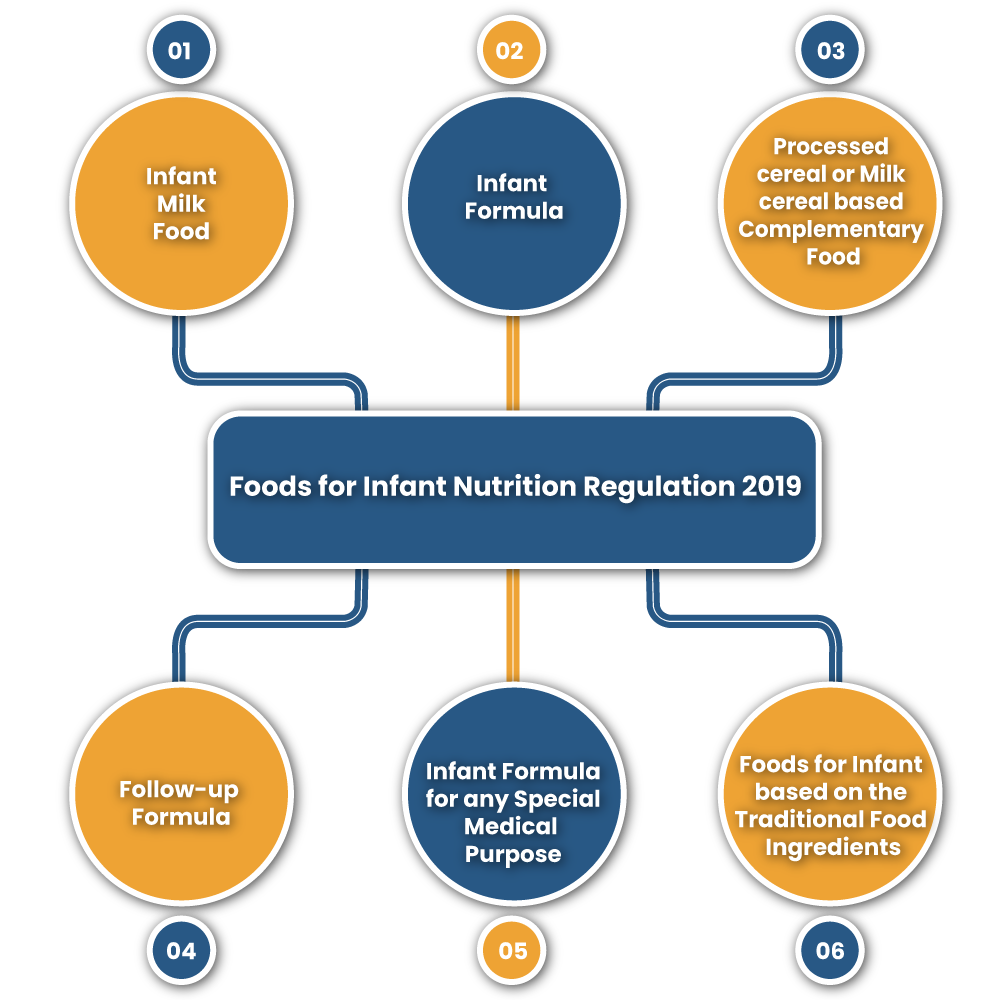

Infant Milk Food
It means a substitute of breast-milk specially manufactured to meet the nutritional requirements of an infant.
Infant formula
It means a substitute of breast milk specially manufactured by the product based on milk of buffalo or cow or mixture of other ingredients that are suitable for infant feeding in order to meet nutritional requirements for an infant.
Processed Cereal or Milk cereal based Complementary Food
It means the food made up of milk, cereals, nuts millets, and legumes (pulses). It includes food made of protein concentrates or protein isolates or by defatted edible oil seed extracts as prepared that gets dissolved with water or milk easily.
Follow-up Formula
It means the food intended for use as a liquid part of the complementary diet for infants when prepared as per the instructions for use.
Infant Formula for any Special Medical Purpose
It means for a human milk substitute or infant formula which is manufactured specially to meet the special nutritional requirements for infants having any disease, specific disorders, or medical conditions.
Foods for Infant based on the Traditional Food Ingredients
These are the products which are prepared traditionally at home for feeding infants but have been processed and provided in packaged forms.
What are the General Requirements for Infant Nutrition as per Guidelines?
- Foods for infant nutrition has to be packed in hermetically sealed, clean and sound containers or in flexible pack made from paper, polymer or metallic film in accordance with the Food Safety & Standards (Packaging and Labelling) Regulations, 2011. It must be packed with inert chemicals in order to protect the contents from deterioration.
- The infant formula category for any special medical purposes provided under these regulations must be exempted from the provisions specified under the Regulation of the Food Safety & Standards (Packaging & Labelling) Regulations, 2011. Furthermore in case any ingredients with known allergen city are present then there has to be warning mentioned on the label.
- The infant formula category food for infant based on traditional food ingredients provided under these regulations has been conform to the microbiological standards for milk & milk products of category infant formula as given under Appendix B of the Food Safety & Standards (Food Products Standards & Food Additives) Regulations, 2011.
- The variation of ± 5.0% of the declared values of an added ingredient due to analytical variations must be allowed.
- The food for infant nutrition will use the source compounds for vitamins, minerals and other nutrients from Schedule I (a), Schedule I (b) & Schedule I (c), provided under the regulations.
- The food for infant nutrition must contain algal and fungal oil as source of Arachidonic Acid (ARA) and Docosahexaenoic Acid (DHA) from Mortierella alpina, Crypthecodinium cohnii, Schizochytrium sp., and Ulkeniasp at the level of maximum 0.5 %. The DHA of total fatty acids & ratio of DHA: ARA as 1:1 minimum. The DHA content must not be less than 0.2% of total fatty acids, if a claim related to addition of DHA is made. The Infant Milk substitutes for premature infants must not contain less than 0.2 %. DHA of total fatty acids & ratio of DHA: ARA as 1:1 minimum.
- The person shall not manufacture, store, sell or exhibit for sale, an infant milk food, milk cereal based complementary food, and processed cereal based complementary food with follow up formula except under Bureau of Indian Standard Certification Mark. The infant formula category for special medical purpose provided under these regulations must be exempted.
- Food for infant nutrition must comply with the requirements of the Feeding Bottles, Infant Milk Substitutes, and Infant Foods (Regulation of Production, Supply & Distribution) Act, 1992 which was amended in 2003. In case of infant formula for any special medical purpose when breastfeeding is contraindicated on medical grounds for the disorders, diseases, or medical conditions for which the product is intended, the labelling provision “Mother’s Milk Is Best For the Baby” will not be required.
- Food for infant nutrition has to comply with all the requirements of Legal Metrology (Packaged Commodities) Rules, 2011 only except for the requirement of standard pack size in accordance with second schedule in case of infant formula for any special medical purpose.
Conclusion
FSSAI registration is a very important to obtain and even the small deviation from rules and regulations can cause rejection of the food product and wasting all the efforts of the manufacturer. Hence, expert guidance is the best way to ensure smooth approval process. The experts at Corpbiz know every regulation and procedure for approval of all the food categories. They will assist you at each and every step, so it is advisable to take services and get approval of infant food product as early as possible.
Read our article: FSSAI Restrain Licensing Authorities from Demanding Immaterial Documents from FBOs











