Patent protection is undoubtedly the foremost priority for any organisation because it can keep them ahead of rivals and reaps stable income for years to come. But somehow, some patents stumble upon unexpected litigations that costconsiderable time and money for companies. In this article, we have curated a list of Landmark judgement on the patent that not only created the buzz in the legal domain but also leave a lasting impact on the constitutional framework of the country.
F. Hoffmann-La Roche Ltd vsCipla Ltd.
Two pharmaceutical giants, viz OSI Pharmaceuticals & F. Hoffmann-La Roche Ltd., have filed the petition for a permanent injunction of the drug due to patent violation, rendition of damages, accounts, and delivery against Cipla Ltd. Mumbai.
- Indian drug producer Cipla won the Landmark judgement against Roche in Delhi High court over the generic version of cancer treatment drug Erlotinib. The case was unique as it is the first Patent litigation in the country, which included pricing issues and public interest that prevent evergreening of the patent.
- In 2008, Roche sued Cipla before Delhi High Court[1], appealing that the generic product Erlocip infringes erstwhile Indian 774 patent claiming “Erlotinib Hydrocloride”. However, the trial judge rejects the petition to bestow injunction preventing Cipla from selling a cheap version of Tarceva on account of public interest and ongoing patent proceedings against 774 patent. Cipla’s cheap version of the drug costs around 1/3 of Roche’s patented medicine.
- Roche’s next petition to the Division bench also rejected in light of the trial judge’s findings. Also, the Court imposed a cost on Roche for suppression of information about the subsequently filed application in India (IN/PCT/2002/00507/DEL).
- The said patent application was on Polymorph Form B of Erlotinib Hydrocloride, which faces disapproval in 2008 following the petition filed by Cipla on Section 3d. Cipla claimed that Tarceva corresponds to Polymorphic Form B is more stable and apt for oral dosage than the compound available in 774 patent consist of a mixture of Form A and B.
- Before the Supreme Court (SC), Roche’s next petition against an order issued by the division bench got disapproved due to ongoing trial at the Delhi High Court.
Read our article:Landmark Judgments on IPR Law in India
Novartis AG vs Union of India
Yet another Landmark judgement of the patent in India created a lot of buzz in the drug manufacturing domain. This time Swiss Drug manufacturer Novartis faces rejection from the patent authority in the light of ‘unconstitutional’ patent law practices.
The Swiss giant seeks a patent for its cancer drug, Glivec, but the government revoked their appeal on the ground of the following facts:
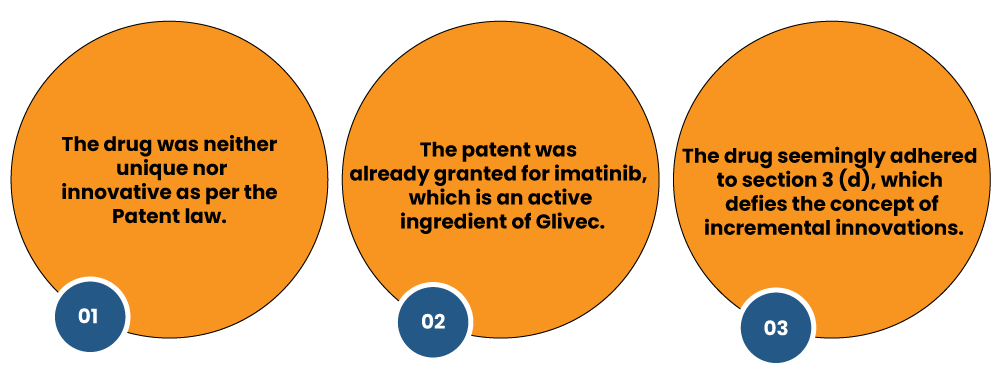

Consequently, the Swiss manufacturer decided to sue the constitutionality of section 3(d) in the High Court, which was rejected in due consideration of the above grounds.
Bajaj Auto Ltd. vsTvs Motor Company Ltd
The case of Bajaj vs. TVS Motor talks about the disagreement related to the unauthorized patent’s application of the DTSi (Digital Twin Spark Ignition). The case is vital on the grounds of the parties’ financial stakes and application of the pith & marrow doctrine. This case is unique as it takes Doctrine of Equivalent into account. Above all, the principles available in finalizing the decision are also crucial.
Glochem Industries Ltd vsCadila Healthcare Ltd
In a recent judgement, the Bombay High Court took validity of pre-grant opposition into account and announced that a new form of a prevailing substance could not come under the patent’s regime unless its claims surpass prior art to ensure the different or enhancing effect.
- Glochem Industries filed a petition before the Court after its pre-grant opposition to Cadila’s patent application was disapproved by the patent authority. Glochem’s primary concerns were that the patent claim didn’t adhere to novelty and lacked enhancing therapeutic effect, as per section 25(1) and Section 3(d) of the Indian Patents Act.
- According to Glochem, Cadillac had failed to provide substantial evidence before Court that the new form of substance enhances the therapeutic efficacy. The company also claimed that the disapproval of the patent’s controller’s opposition resulted from misconstruction and misapplication of the provisions under Section 3(d) of the Act.
This conflict revolves around the substance called clopidogrelbesylate, a salt derived from clopidogrel, which is already patented in the United States.
After prolonged deliberations, Bombay High Court opined that the Golcem’s erstwhile actions to restrain the opposition could act as grounds for litigation. Further, the Court cited that the alleged misrepresentation by the patent controller could solidify the case.Hence, the Court decided to hold the order and directed the authority to reconsider the matter.
Strix Ltd v Maharaja Appliances Ltd
The Delhi High Court granted temporary prohibition on the marketing and manufacturing of Maharaja Appliances’ electric kettle Model No EK 172 due to infringement of Strix’s patent (IN 1,92,511, US 6,080,968). The decisions were based on the following observations made by the Court on why the Indian manufacturer failed to discharge its burden of raising a ‘creditable challenge’ to Strix’s patent validity.
The defendant failed to present some acceptable scientific material, supported by the expert’s explanation that the patent is prima facie susceptible to cancellation.The burden of proof was greater on the defendant as there was no history of Strix’s patent being challenged in the Court.
Bayer Corporation &Anrvs Union of India &Ors
Yet another Landmark judgement that has resonated strongly in the legal domain for years. Instead of filing a petition for infringement, Bayer Corporation filed a writ appeal in the Delhi High court requesting an injunction on Cipla’s marketing approval application in light of the violation caused by their version of the drug, viz SORANIB.
This appeal was one of its kind because it links drug approval to patent infringement in India.
In response, The Delhi High Court refused to stamp their approval on such appeal and eventually imposed a cost of Rs 6,75,000 on the plaintiff to prevent future occurrence of such actions.
- Bayer argues that section 2 of the Drugs and Cosmetic Act, in association with Section 48 of the (Indian) Patent Act, 1970, set up a patent linkage mechanism that prevents anyone from taking marketing approval for a drug over a patented drug. The company also claimed that the CIPLA’s “SORANIB” is a “Spurious Drug” as mentioned under the Drugs Act.
- The High Court of Delhi, in its judgement, clarified that the Drug- Patent Linkage mechanism is an impractical thing as both the Act excels on specific objectives and rights to check the patent standard is with the regime of patent’s controller. Furthermore, such linkage may have a drastic impact on the nation’s public health policy.
- The Court further added that market approval of drug has nothing to take with patent infringement. Hence, patent infringement cannot come to effect unless it is established in a court of law. Such adjudication is beyond the scope of Drug Authorities’ jurisdiction. This is indeed the Landmark judgement when it comes to legal and judicial complexity.
Conclusion
Securing the patent in India is somewhat challenging, considering leverage available within ourlegal framework. The Landmark judgements above have made it evident that clarity on the applicable law is imperative for the plaintiff before filing petition against the the defaulter for patent protection.
Read our article:List of Important Judgments under Trademark that You Must Know!