MedTech Mitra is a pioneering platform that supports upcoming medical technology talent by aiding their knowledge, research, and reasoning and offering crucial assistance in gaining regulatory approvals. It is an endeavour to support healthcare innovations and enable innovators in the medical technology industry.
The MedTech Mitra portal will be coordinated by the Central Drugs Standard Control Organization (CDSCO) and the Indian Council of Medical Research (ICMR) under the patronage of NITI Aayog’s Atal Innovation Mission. Let us discuss how MedTech Mitra is set to transform the medical sector through tech innovations.
What is MedTech Mitra?
Dr Mansukh Mandaviya, Union Minister of Health & Family Welfare, and Chemicals & Fertilisers, officially introduced MedTech Mitra, a ground-breaking platform designed to develop the potential of young innovators in the medical technology sector.
The strategic endeavour is intended to offer MedTech innovators all-encompassing support, helping them refine their knowledge, research, and reasoning and assisting with regulatory clearances for their healthcare solutions.
The Indian Council of Medical Research, under the direction of NITI Aayog, is working in collaboration with the Central Drugs Standard Control Organisation (CDSCO) to expedite the development of accessible and reasonably priced indigenous medical devices and in-vitro diagnostics.
MedTech innovators will receive strategic handholding support for clinical evaluation, regulatory facilitation, and new product uptake.
Facts about Medtech Mitra
Medical technology, or MedTech, refers to a wide range of advancements in healthcare, including pacemakers, MRI scanners, insulin pumps, and surgical equipment. A brief introduction of MedTech Mitra is as follows:
- Initiative Launch: The Union Minister of Health and Family Welfare introduced MedTech Mitra to support MedTech inventors and improve healthcare solutions.
- Objective: MedTech Mitra aims to help young people develop their research, expertise, and reasoning while guiding and supporting them in getting regulatory approvals.
- Strategic Collaboration: The platform works with prospective MedTech entrepreneurs, offering vital assistance to help them refine their concepts and successfully handle regulatory procedures.
- Progress in the MedTech Sector: The MedTech sector has made impressive strides through investments in medical drug parks, production-linked incentive programmes, and research programmes.
- Reducing Import Dependence: The cooperative project aims to lessen the reliance on imports for medical equipment by supporting the domestic development of reasonably priced, superior MedTech gadgets.
- Key Policies and Incentives: The adoption of MedTech research policies and incentive programmes is essential to promoting quality and innovation in the medical technology industry.
- National Impact: MedTech Mitra reinforces India’s goal of self-reliance by supporting domestic manufacturing of vital medical equipment and nurturing local talent.
Scope of Facilitation by MedTech Mitra
Promoting product development through collaborations between engineers and clinicians. It’s scope extends to the following avenues:
Handholding Support
- Regulatory facilitation
- Pre-clinical/Clinical evaluation
- Guidelines & HTA (health technology assessment)
- Uptake of new products
Information cell
- Applicable standards
- Testing and manufacturing facilities
- Funding opportunities
- I.P. advisory
Pillars of Strengths for MedTech Mitra
Regulatory Strategy
Collaborating with CDSCO, the National Regulatory Authority, to streamline the regulatory approach.
Key Facilitator
NITI Aayog-Atal Innovation Mission oversees the ICMR-Medical Device & Diagnostic Mission Secretariat (MDMS).
Pre-compliance Gap Analysis & Testing
- Trusted knowledge partners
- Bureau of Indian Standards (BIS)
- Kalam Institute of Health & Technology, Vishakhapatnam (KIHT)/Andhra Pradesh MedTech Zone (AMTZ)
Policies & Guidelines
DHR-Centre provides evidence-based guidelines for healthcare in India.
Pre-clinical Evaluation
- Cutting-edge facilities with proven pre-clinical study skills
- ICMR-National Animal Resource Facility for Biomedical Research, Hyderabad
- ICMR-DHR-Large Animal House Facility at AMTZ
- ICMR Institutes
Health Technology Assessment
DHR-Health Technology Assessment in India (HTAIn cell), with its unique ability to assess cost-effectiveness and suitability
Clinical Evaluation
Indian Clinical Trial & Education Network (ICMR-INTENT)-Pan-India is a network of 47 clinical facilities, hospitals, and medical schools with a track record of conducting clinical research.
Motive of MedTech Mitra
MedTech Mitra aims to foster medical technology entrepreneurs in developing accessible, low-cost domestic medical devices and in-vitro diagnostics through strategic support for clinical evaluation, regulatory facilitation, and adoption of innovative products. MedTech Mitra’s Facilitation Services intends to nurture new product development by uniting medical professionals and engineers.
The Indian Council of Medical Research (ICMR), an independent organization in charge of planning, directing, and carrying out biomedical research in India, is subordinate to the Department of Health Research (DHR). The Central Drugs Standard Control Organisation (CDSCO), a Ministry of Health & Family Welfare’s Directorate General of Health Services division, is the Indian equivalent of the National Regulation Authority (NRA).
Medtech Mitra and CDSCO registration are somewhat similar in terms of their intent. Both ensure that medical devices meet the necessary safety and productiveness standards.
Innovator support offers medical technology entrepreneurs full support by assisting them at every development stage, from concept to clinical product assessment. It includes:
- Regulatory Facilitation: Assistance in securing the required approvals and guaranteeing adherence to appropriate standards and rules while streamlining the regulatory procedures for MedTech products.
- Technology Uptake: Promoting a broader adoption by making it easier for new MedTech products to be integrated into healthcare procedures and systems.
- Collaborative Coordination: Guaranteeing an efficient platform to implement, supervise, and coordinate efforts between the Central Drugs Standard Control Organisation (CDSCO) and the Indian Council of Medical Research (ICMR).
- Guidance under NITI Aayog: Operating under the direction of NITI Aayog’s Atal Innovation Mission to help innovators align with national innovation plans and guarantee a unified strategy for the advancement of medical technology.
- Boost to Domestic Manufacturing: Helping the medical device industry thrive by encouraging domestic manufacturing through programmes like production-linked incentive schemes.
- Self-Reliance: Promoting domestic development of reasonably priced, superior medical technology products and diagnostics to lessen reliance on imported medical equipment.
- Capacity Building: Enhancing the capacities of medtech experts and innovators by offering opportunities for skill development, training, and instructional materials.
- Economic Impact: Promoting employment possibilities by encouraging innovation and entrepreneurship and assisting in the MedTech industry to boost overall economic growth.
- Alignment with National Health Goals: Supporting tech developments to contribute to better healthcare delivery, cost, and accessibility in line with broader national health objectives.
India’s Holistic Approach to Health
In line with Viksit Bharat’s mission, Dr Mandaviya underlined the medtech industry’s critical role in India’s healthcare system. While acknowledging the industry’s 80% reliance on imports, he also emphasized the noteworthy advancements made possible by MedTech research regulations, investments in medical drug parks, and production-linked incentive programmes. MedTech Mitra’s collaborative approach is anticipated to encourage domestic production of reasonably priced, superior medical gadgets, lowering the country’s overall import dependency.
The connection between BIS certification and Medtech Mitra lies in their objective of maintaining safety and compliance standards for medical devices in India.
Role of MedTech Mitra
Innovators must overcome challenges and obstacles related to the commercialization of their ideas.
This is where MedTech Mitra plays a pivotal role. It gives upcoming businesses the required tools to simplify their research and development and offers various other services to help achieve the national goal of Atmanirbhar Bharat.
According to the Union Minister for Health and Family Welfare, MedTech Mitra is an ecosystem and community that brings about revolutionary change rather than just a platform.
It aims to harness the power of indigenous technologies, which are not only precise but affordable, to transform the country’s healthcare sector.
MedTech Mitra is an ambitious project to unite various stakeholders in the medical technology space, foster innovation, and improve cooperation within the healthcare sector.
India’s Holistic Approach and Vision for the Future
The medical device industry is a significant funding source for India’s healthcare system. However, India is adopting a holistic approach to health to change the country’s health environment by 2047, which is in line with the Viksit Bharat goals.
Union Health Minister emphasized the significant advancements made possible by production-linked incentive programmes, investments in medical drug parks, and the adoption of MedTech research policies despite the industry’s 80% reliance on imports.
MedTech Mitra’s cooperative strategy will notably reduce the country’s need for import by enabling it to produce reasonably priced, superior medical devices domestically.
Dr Mandaviya projects that India’s medtech industry will generate $50 billion in sales by 2030. India has seen a rapid shift due to technological breakthroughs, including virtual reality, robotics, artificial intelligence, big data, and nanotechnology, and this goal seems quite achievable.
The emphasis lies on proactive support at the approval stage, which is essential to achieving India’s goal of self-sufficiency.
In cooperation with regulators and other essential stakeholders to give strategic support, MedTech Mitra will respond to all the questions raised by innovators and offer tailored guidance through a meeting (as soon as possible).
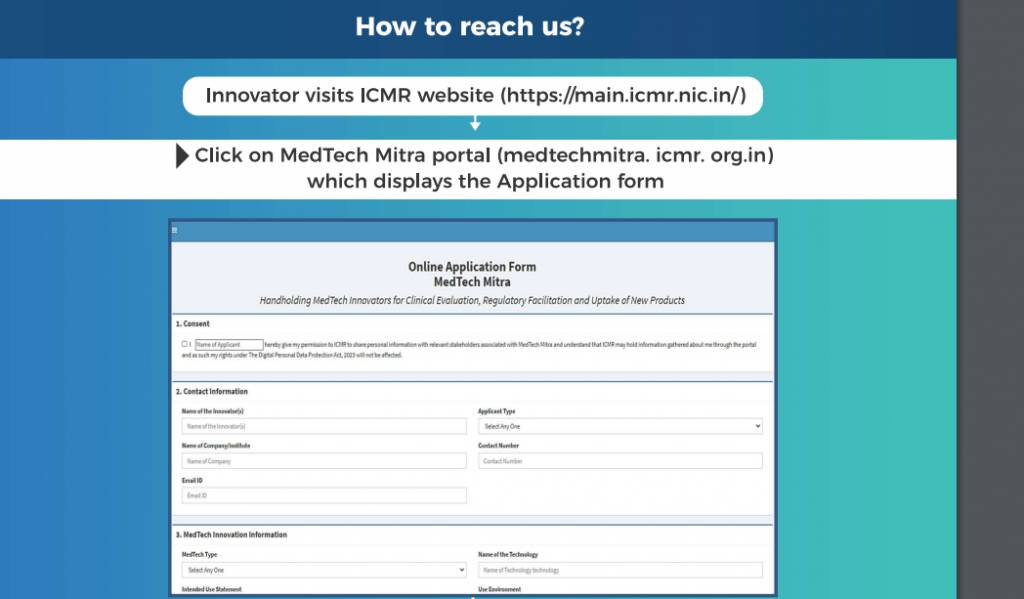
The applicants must note that as only 5–6 innovator spaces are available per meeting, they must make reservations to book their slots. Innovators are advised to submit applications through the MedTech Portal to schedule appointments in advance.

Significance of MedTech Mitra
Let us look at the importance of MedTech Mitra below:
- Innovation Catalyst: MedTech Mitra promotes innovative solutions by catalyzing innovation in the medical technology industry.
- Regulatory Streamlining: The project lowers barriers for MedTech entrepreneurs and speeds up product approvals by streamlining processes and providing regulatory help.
- Collaborative Coordination: The joint venture between NITI Aayog, CDSCO, and ICMR guarantees a coordinated strategy by using each organization’s advantages.
- Domestic Manufacturing Boost: It aims to help the government promote local manufacturing by combining the goal of offering incentive programmes and assisting indigenous growth.
- Self-Reliance in Healthcare: MedTech Mitra seeks to reduce import dependency by strengthening the nation’s independence in vital healthcare technologies and lowering external vulnerabilities.
- Capacity Building: The platform enhances the abilities of MedTech experts through training and capacity-building programmes, guaranteeing a skilled workforce.
Conclusion
The launch of MedTech Mitra is an important step forward in India’s healthcare history. It is anticipated to stimulate innovation and bolster the country’s domestic capacity to produce high-quality medical devices. This strategic programme reflects the government’s determination to promote innovation, decarbonize the economy, and steer India towards MedTech self-sufficiency. In addition to helping innovators with clinical evaluations and regulatory compliances, MedTech Mitra articulates entrepreneurs’ difficulties when attempting to commercialize their ideas.
MedTech Mitra is a positive step forward in enabling burgeoning startups to simplify their innovation, support research, and help develop an Atmanirbhar Bharat!
Frequently Asked Questions
What is MedTech Mitra?
Ans. MedTech Mitra is an innovative platform that aims to assist and uplift the medical technology industry in India by offering tools, direction, and a forum for creativity and cooperation.
Who launched MedTech Mitra?
Ans. MedTech Mitra was launched by Dr. Mansukh Mandaviya, Union Minister of Health and Family Welfare and Chemicals & Fertilizers in December 2023.
What is the main motive of MedTech Mitra?
MedTech Mitra is a system of expert help, facilitation, technical support, and regulatory guidance. The Government of India launched this initiative to handhold innovators in clinical evaluation, regulatory facilitation, and the uptake of new medtech products. It aims to shape the research of upcoming medical technology inventors and offer assistance in getting regulatory approvals while providing them with guidance and support.
How MedTech Mitra help lessen India’s reliance on imports for medical devices?
Ans. By encouraging homegrown products at reasonable prices and developing superior medical equipment through joint ventures, MedTech Mitra aims to lessen India’s reliance on imports for medical devices.
What is the value of the MedTech industry in India by 2030?
Ans. Dr Mandaviya forecasts that India’s MedTech industry will be valued at $50 billion by 2030.
How can entrepreneurs apply for assistance from MedTech Mitra?
Startups may apply via the official MedTech Mitra website or platform, where they must submit an application and supply relevant information about their business or initiative.
Who can take advantage of MedTech Mitra?
MedTech Mitra’s tools, advice, and networking opportunities can benefit businesses, researchers, startups, and medical technology firms.
Read Our Article: A Guide On CDSCO Guidelines For Medical Devices In India













