When a pharmaceutical entity first evolved a new drug to be used for disease treatment, in its initial days sold under a brand name, the doctors can specify the drug for use by their patients. The drug gets protection under the regime of Intellectual property, which means that only the pharmaceutical company that holds the patent is permitted to produce, market the generic pharmaceutical drug, and eventually get the benefit from it.
In extreme cases, the drug patent is granted for around twenty years in India. The period of the patent varies between nations and also between the drugs. Since the company filed for a patent long before the scientific experiment to evaluate a drug’s protection from harm and adequacy has begun, the influential patent duration after the drug has finally approved is often around seven to twelve years.
What Is Generic Pharmaceutical Drug?
A generic equivalent drug is one that performs the same way as a brand-name drug. In an aspect, it stimulates the original generic pharmaceutical drug in dosage, strength, usage, quality, manages technique, and performance.
All these analogies assist the generic pharmaceutical drug to work the same way the original did, as it is biologically similar to it. Further, generic medicine should also do the same medication purpose. Further, they have to give the same advantages as brand-name pharmaceuticals.
What Are The Label Of Generic Medicines In India?
The below-listed are the generic pharmaceuticals medicines exist in India.
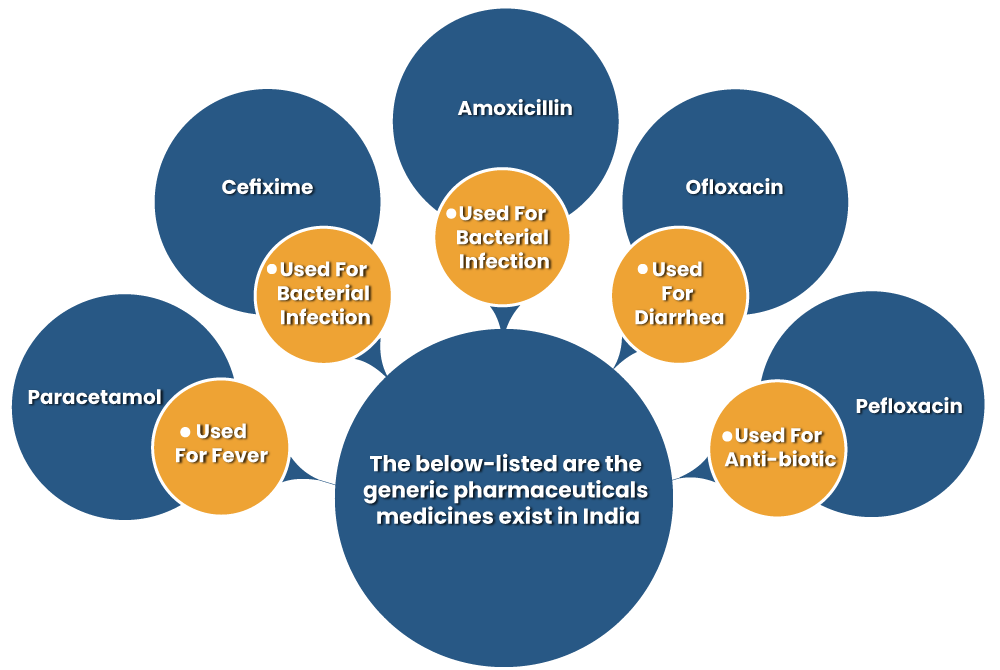

Generic Pharmaceutical Drug Works Similar to Brand-Name Drugs
A generic pharmaceutical drug performs in the same way and gives the same medical advantages as its brand-name version. This standard administers to all Foods & Drugs Administer (FDA) authorized generic pharmaceutical drugs. A generic pharmaceutical drug is the same as a brand-name drug in dosage, effectiveness, safety, strength, stability, quality, and the way it is consumed and should be used.
The FDA Generic Pharmaceutical Drugs set up directs a severe analysis to ensure generic pharmaceutical drugs satisfy these conditions. The FDA also administers 3,500 production plants a year, assuring compliance with the authority regulations on good manufacturing practices.
The FDA team also regularly monitors generic pharmaceutical drug products to prepare certain medicines at all supply chain levels, from active pharmaceutical ingredients (API[1]) to products being sold to consumers, safe, effective, and of high quality.
Generic Pharmaceutical Drugs Must Satisfies High Standards to Get FDA Approval
Food & Drugs Administration (FSA) needs drug companies to establish that the generic pharmaceutical drugs can be efficiently counterfeited and give the same medical advantages as the brand-name medicine that it duplicates. The compressed new drug application (ANDA) acknowledged by drug companies must show the generic pharmaceutical drug is the same as the brand-name version in the below said ways:-
- The generic pharmaceutical drug’s active element is the same as in the brand-name drug/discoverer drug.
- The generic pharmaceutical drug has the same strength, use indications, form (such as a tablet or an injectable), and route of effort (such as oral or topical).
- The inactive elements of the generic pharmaceutical drug are acceptable.
- The generic pharmaceutical drug has been produced under the same strict conditions as the brand-name medicine.
- The holder in which the medicine will be shipped and sold is suitable, and the label is the same as the brand-name medicine’s label.
Authorized Generic Medicines are Only Sold after Patent Registration and Particularly Protecting the Brand-Name Version End
- Patent registration and particularities are prepared for protection for drug makers that may impact how and when a generic pharmaceutical drug is approved and sold. New brand-name drugs usually get protection under the regime of IPR that stop others from selling generic versions of the same drug. Duration of marketing particularity for brand-name drugs can also affect the approval of generic pharmaceutical drugs.
- FDA must adhere to the delays in authorization that the patents and particularities impose. When these patents and retailing particularities expire or the generic drug company successfully challenges the patents, the generic pharmaceutical drug can get full approval and be sold.
Drug can be Produced as a Generic Pharmaceutical Drug
When the patent has expired, the drug can be mass-produced and sold by other companies. At this point, the drug is arbiter to as a generic pharmaceutical drug. Under the guidelines in many countries, generic pharmaceutical indistinguishable drugs have to be identical to the branded drug in terms of adequacy, safety, usage, route of drug administration, pharmacokinetics and, pharmacodynamics.
Therefore, a drug can be produced as a generic pharmaceutical drug when the following condition satisfied.
- The patent has been expired.
- The company that would produced the generic pharmaceutical drug accredit that the patents held on the drug are either void, are invalid or would not be violated upon
- There has never been any patent hold on the drug before
- In countries where there is no provisions exist related to drug patent protection
When the generic pharmaceutical drug is on the market, the ownership owned exclusively of the patent holder is evacuated. This stimulated competition and appeared as a critical fall in drug costs, which assures that life-saving and important drugs reach the general population at comparative prices.
The company have the prior patent may, however, renews the patent by forming a new version of the drug that is significantly changed compared to the original compound. However, this may need new medical trials and re-application of the patent. Furthermore, the new product may have to compete with the original generic molecule on the market, unless the drug regulators find faults and evacuate the original from the market altogether.
Why are Generic Pharmaceutical Drugs in India Considered Too Bad?
Indian generic pharmaceutical drugs and the generic pharmaceutical market in India have several quality and safety concerns. In year 2013, Ranbaxy solicited guilty to various charges regarding the sale of adulterated drugs.
Further, the discredited pharmaceutical company had to pay penalty of sum of amount $500 million for the same as they were entangled manipulating to test results to fast track production. This case also influence many to believe that India’s generic pharmaceutical market produced low-quality and inadequate generic pharmaceuticals drugs.
While these companies manufacture good quality drugs for export to other nations as they have strict policies, they sell substandard drugs in India. Similarly, it is as they felt they produced more effective than generic pharmaceutical drugs. Therefore, the aspect, capability, and conditions of generic drugs in India are a growing concern for the public and the government.
Conclusion
New drugs have to undergo severe testing and authorization process before they get drug patents in India. Moreover, once they get drug license over their product, they become the only company granted to produce, and retail that drug.
When the patent got expires, other companies can retail generic pharmaceutical drug versions of the same drug, via a more straightforward authorization procedure. Further, the generic pharmaceutical drug includes the same amount of active elements but may have distinct fillers. Therefore, while they may have the same affect, the way they work and get absorbed may be different.
Generic pharmaceutical drugs that assist to prevent convulsion must be absorbed into the blood in the right quantity to be active. Therefore, people who do well on one generic pharmaceutical drug may not do as well on another. The same posses when people switch from a brand-name drug to a generic pharmaceutical drug. When different companies produce the same generic pharmaceutical drug, they may avail various fillers, leading to a difference in absorption and potency. Therefore, this can give advantage to some variations in the amount of absorption, side effects caused, and other such issues.
Read our article: How can one have the Right to Buy Pharmaceutical Drug?













