To ensure the good health of a nation, access to medicines and drugs are indispensable. However, access to medicines and drugs should be controlled to ensure that individuals do not misuse such essential drugs.
Therefore, the government has laid down strict laws and regulations for the production and distribution of drugs in India. Manufacture and sale of drugs are regulated by the Laws and Regulations given in the Drugs and Cosmetics Act, 1940. The production and distribution of drugs in India can be done after obtaining the drug license. The individual needs to submit the list of particulars discussed below to get the drug license.
Registration Process of Drug License
Registration of Drug Licenses and everything associated with drugs is governed and controlled by the Drugs and Cosmetics Rules 1945. The act controls every aspect of the drugs, from raw material procurement during production to distribution and sale, until it reaches the customer or patient. Drug license is important for all sorts of drugs, cosmetics, medicines businesses, including Homeopathic, Allopathic, Unani or Ayurvedic drugs, etc. This Act even deals with Pharmacist, hospital, or a dispensary regarding usage of drugs. The act also involves the Ayurvedic and Unani drugs in it.
Read our article:What Are the Conditions Required To Obtain Drug License under Drugs & Cosmetic Act, 1940
Classification Of Drug License
The classification of drug license depends on the nature of pharmaceutical business. There are primarily 2 categories of drug license usedto obtain the drug license i.e, drug distribution or sale in India. But, overall, there are 7 types of Drug License authorized in India.
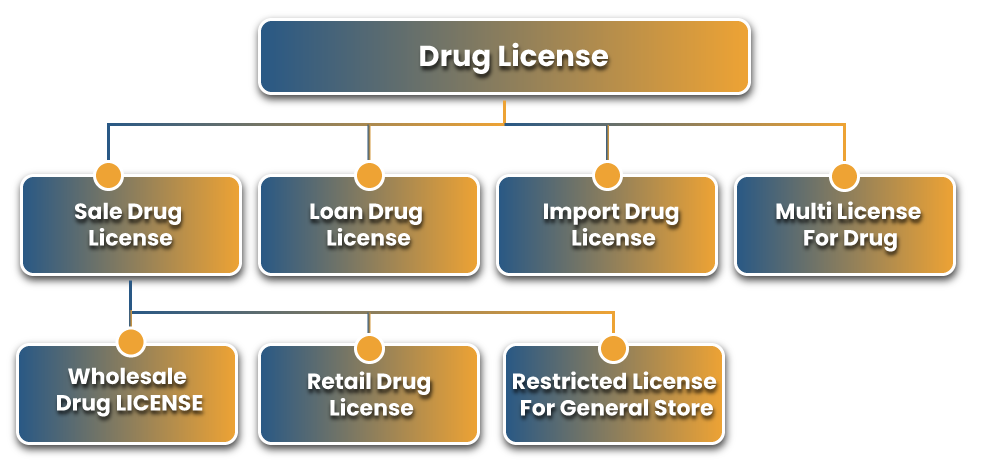

Retail License
This license is authorized to Individuals/ agencies involved in the retail business of the drugs.
Wholesale Drug License
This License is granted to Individuals/ agencies involved in the Wholesale distribution or sale of drugs. The License in India is granted by the Central Drugs Standard Control Organization (CDSCO) to those wholesalers involved in the business of pharmaceuticals.
Manufacturing License
Manufacturing drug Licenses must be obtained by the manufacturers of Ayurvedic, Allopathic, Cosmetics products, or any other drugs under the Drugs and Cosmetics Act, 1940[1]. The concerned State Government grants this License to the applicant.
Loan License
This License is got by those industrialists who do not have their own land or property for the production of their drugs but want to manufacture the drugs with their brand-name on land or property of those who are already allowed to do so with the License of manufacturing.
Restricted License for General Store
This Restricted License is granted under Forms 20A and 21A to the respective dealers to sell drugs without the supervision and control of any qualified person.
Import Drug License
Import Drug License must received by those entrepreneurs who are availing imported products for the production of drugs or those involved in the sale or distribution of imported drugs in India.
Multi License for Drugs
This License must be obtained by those industrialists who are doing business activities in more than one State. No one can sell drugs at more than one location or state without getting this Multi-Drug License
Forms for Drug License
The below mentioned are the forms needs to be filled by the applicant at the time of registration of drug license
Form 19
Form 19 is authorized to renew of a license to sell, stock, exhibit or offer for sale, or distribute drugs apart from those specified in Schedule X.
Form 19A
Form 19 A is authorizing for renewing a restricted license to sell, stock, exhibit or offer for sale, or distribute drugs by retail via dealers who don’t involve the service of a qualified individual.
Form 19B
Form 19 B is authorized for license to sell stock or exhibit or offer for sale, or distribute Homoeopathic Medicines.
Form 19C
An application for authorizing or renewing a stock license, sell, offer for sale or exhibit, or distribute drugs mentioned under the Schedule X.
Form 24
An application is for either authorization of a license or for the renewal of a drug license for distribution of drugs/medicines or to manufacture for sale other than those which are specified under Schedule C, C (1) and X.
Form 24A
An application is for either the authorization of a loan license or renewal of a loan license to distribution of drugs/medicines or manufacture for sale other than those specified under Schedule C, C (1) and X.
Form 24B
An application is for authorization or renewal of a licensee to repack for sale or distribution of drugs/medicines, being drugs other than those specified under Schedule C and C (1) excluding those specified in Schedule X.
Form 24C
An application is for the authorization or renewal of a license to manufacture for sale or distribution of Homoeopathic medicines.
Form 24F
Application is authorized for renewal of a license to manufacture for sale or for distribution of drugs specified under Schedule X and not specified in Schedule C and C(1).
Form 27
Application is authorized or renewal of a license to manufacture for sale or for distribution of drugs specified under Schedule C and C (1) excluding those specified in part XB and Schedule X
List Of Particulars For Drug License
The below discussed are the list of particulars asked to submit by the applicant at the time of drug license registration.
List Of Particulars For New Retail Drug License
(Drug Licence No. 20, 21)
- Form No. 19
- Fee challan Rs. 3000/-
- Affidavit duly signed by applicant on stamp paper of 20 Rs.
- Educational certificate copies of applicant (Self attested)
- Self attested copies of Identity proof of applicant ( Domicile/ Driving license/ Voter ID card)
- Affidavit duly signed by registered pharmacist.
- Educational certificate copies of registered pharmacist (Self attested)
- Self attested copies of Id proof of registered pharmacist (Voter ID card /Domicile/ Driving license)
- Blueprint of proposed area for performing business activities.
- Electricity bill of proposed area for performing business activities.
- Copy of Refrigerator bill (Proof of Existence of Refrigerator to preserve the Drugs)
- Rent agreement of the premises
- Property document justifying the ownership of that premises including the copy of tax receipt of proposed area for performing business activities.
- Covering letter along with the statement of purpose of business.
- Photo 5-5 each of Regd. Pharmacist and applicant.
List Of Particulars For New Wholesale Drug License
(Drug License No. 20B, 21B)
- Form No. 19
- Fee challan Rs. 3000/-
- Affidavit of applicant on stamp paper of 20 Rs.
- Educational certificate copies of applicant (Self attested)
- Self attested copies of Identity proof of applicant (Voter ID card /Domicile/ Driving license)
- Affidavit of Competent person.
- Academic certificate copies of Competent person (Self attested)
- Identity proof of Competent person (Voter ID card/ Domicile/ Driving license)
- Work experience certificate of competent person.
- Electricity bill of proposed area for performing business activities.
- Copy of Refrigerator bill.
- Rent agreement
- Property document which justifies the ownership of the premises including the copy of tax receipt of the proposed area for performing business activities.
- Covering letter along with a statement of purpose of business.
- Photo 5-5 each of competent person and applicant.
List of Particulars Required For the Issue of Wholesale Drug License
- Form Number— 19
- A letter declaring the purpose of acquiring the Wholesale Drug License
- Receipt of Property Tax
- Challan of fees of the amount of Rs. 15,00/
List of Particulars Required For the Partnership Firm for Obtaining Wholesale Drug License
- List of Partners
- Partnership Deed
List Of Particulars Required For The Public And Private Company For Acquiring Wholesale Drug License
- Certificate of Registration.
- Memorandum of Association (MOA)
- Article of Association (AOA)
- Appointment of the authorized person who will be responsible for daily affairs and operations.
List of the Directors and Shareholders
- An Affidavit from the Directors
- Photograph, ID, and Address Proof of the Directors
- Cancelled Cheque and PAN Card
- Copy of the Board resolution regarding the authorized person’s appointment, whowill be responsible for daily affairs and operations.
List of Particulars Required for the Pharmacist
- Bio-data of the Competent Person as per Performa
- Certificate of Experience
- Affidavit of Pharmacist
- Registration Certificate issued by the Pharmacy Council
- Details of Educational Qualification
- Photograph, ID, and Address Proof of the Competent Person
- Appointment Letter of the Competent Person
List of Particulars Required For the Medical Shop for Drug License
There are two options to get the drug license for the medical shop is as follows;
For Self-owned Property
- Registered Sale Deed
- Registered General Power of Attorney
- Document of transfer property in the name of the owner
- For unregistered GPA, unregistered Sale Deed, it must be annexed with the Property Tax Receipt, Water Bill, Electricity Bill.
- In the case of Khasra, Khatauni will be annexed.
For Rented Premises
- Registered Rent Agreement with the Rent Receipt
- In the case of Unregistered Premises, the Agreement must provide for owner documents.
- Owner of the Premises has to provide a No Objection Certificate (NOC)
- Blueprint of the Premises
Concluding Remark
We are aware that the government is working for us and can ensure public safety at large. Considering the public interest, the concerned government has prepared and established various guidelines to ensure and secure every individual health.
The drugs used for medical purposes need to be observed under the influence of central authority; otherwise, an enormous supply of drugs can push our society in hazardous and threat to humans’ endangered lives. We must follow the central government’s norms to benefit from such life-saving drugs in an organized way. It’s the duty and responsibility of every individual towards their Community. Kindly associate with Corpbiz expert to know more about the particulars demanded at the time of registration of drug license.
Read our article:A Complete Guide to Start a Medical Store Business in India













