The statement concerning Health Supplement, Nutraceutical, Food for Distinct Dietary Use, Food for Distinct Medical Persistence, Purposeful Food, and Fresh Food has been public with the Food Safety and Standards Regulation of India,2016 in FSSAI on 23rd December 2016 Official Gazette. When this regulation is issued in the Authorized Journal, its determination came into strength.
The compliance of these guidelines has been made obligatory for the food business operatives by FSSAI. FSSAI Authorization for food Supplement business can be attained online from official website that is fssai.gov.in. It is substantial to note that FSSAI guidelines cover only 8 food categories and carry out info like arrangement, tags, privileges, etc., whatsoever is essential.
These food products are as follows:-
- Health Supplements
- Foods for Special Dietary Use
- Nutraceuticals
- Speciality Food containing plants or botanicals
- Food for Special Medical Purpose
- Foods Containing Prebiotics
- Foods Containing Probiotics
- Novel Foods
FSSAI Rules for food supplement business
In current years the FSSAI Rules for food supplement business have been modified, keeping in mind health issues and inferior supply of food supplement business in India. The food crops under this group can be shaped, factory-made, and wholesaled in the procedure of tablets, capsules, sauces, and more.
The food products must achieve superiority standards and conditions, as stated in British Pharmacopoeia, Indian Pharmacopoeia, or United States Pharmacopoeia. In India, as a Food Business Operators, you can apply for Basic FSSAI License if your revenue is less than INR 12 Lakhs, State license in case your revenue is More than INR 12 Lakhs and less than INR 20 Lakhs.
It is important that food products and their preparation must be founded on sound nourishment and curative ideologies and should be maintained by scientific data that has been suitably authenticated. A modest mixture of raw materials and vitamins framed in the procedure of tablets, drugs, or sauce cannot be measured as food products unless a proper food arrangement is trailed by addition precise reserves and vitamins. The food products should not comprise steroids, hormones, or any psychotropic constituents.
The number of nutrients that are added to the food products must not overdo RDA explained and stated by the (ICMR) Indian Council of Medical Research. If the requirement concerning these standards is not precise, the Codex Alimentarius Commission ethics should spread over. Permitted extracts and ensigns may be used by the Food Business Operators by way of acceptable in Schedule VF rules. Food Safety and Standards (Food Product Standards and Food Additives) Guidelines, 2011 to permit nature-identical, natural, or synthetic flavors to be used.
FSSAI Labelling Requirements for Food supplement Business
There are specific guidelines concerning labeling on the food products as per the Food Safety and Standards (Packaging and Labelling) Regulations, 2011[1], and additional detailed category supplies mention on each food product. The tags necessity offer the following data: If you are in a preliminary stage, a Food Supplement business in India, and then you should see the FSSAI Labelling condition before obtaining for Food License.
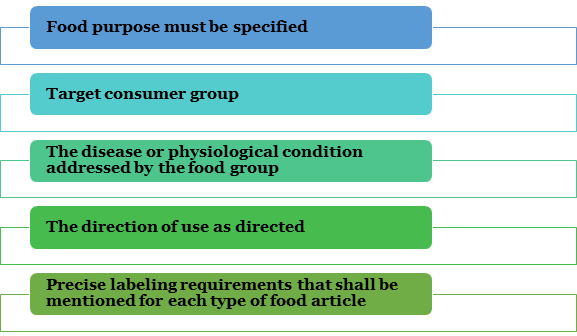

Necessary info must be provided on the associated leaflet or label or additional announcement or classification on each type of food product as per guidelines regarding the purpose and nature of food article along with the specific protections and directions regarding its usage.
A food product that has not remained changed in any way and is effortlessly appropriate for usage as per a precise nutritional régime for its accepted arrangement shall not be careful as “special dietary,” “health supplement” or “special dietetic.”
The food product will come with a declaration on its label explanation of the nature of the food ( take it as X food products). Here X food products describe the requisite characteristic as recognized by mechanical data that is usually acknowledged. Maintenance must be occupied that the declaration is not at all deceptive for the purchasers.
Read our article: Food Safety and Standard Act– Everything a restaurant owner should know
FSSAI Rules for Food Supplement Business related to Additives & Product Elements
Pure and detailed timetables division procedures with thorough necessities where info about the use of reserves, vitamins, botanic elements, amino acids, food seasonings, nutraceutical constituents, prebiotics as well as probiotics is public.
There is a list of four hundred parts of botanically source plants in one of the agendas that are permitted to be used as food constituents that are covered in the guidelines. As per the regulatory provisions, the elements that have been specified in schedules from Ist to VIIth may be used. Similarly, following the above rules, the flavors can also be used as practical to the groups specified in Schedule VA to VF.
FSSAI Rules for Food Supplement Business or Sale of Health or Nutritional Claims
FBOs can make nutritional or health claims for constituents that have been quantified in Schedules I-VI. The health privileges can be made dependent upon Benefits to the health of a person and Nutritional or nutrient ingredients.
The following can be involved in the health rights made regarding food:-
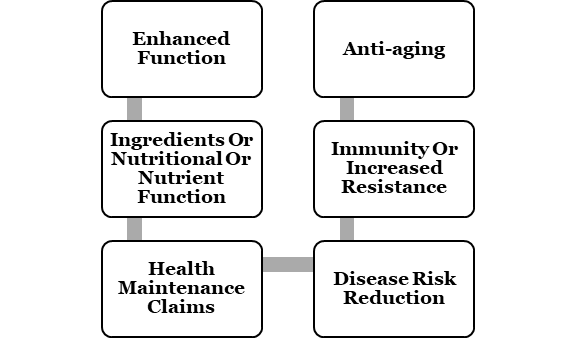

It is crucial to authenticate health privileges made by Food Business Operators with the help of precise and satisfactory certification and human educations directed on the same. In case other privileges are made, it is indispensable to get them approved by the Food Authority. FSSAI strictly prohibits potential privileges concerning curative a diversity of ailments or making worth claims like deterrence of disease or cure of tumor over pictures or by name, symbols or articles, fat profile, or electrocardiogram.
FSSAI Rules for Food Supplement Business in India on condition that some rational Limits on Sale of Certain Food Supplements
The FSSAI also eases limit or restrictions of the auction of particular food products that are obtainable in the marketplace: –
- Foodstuff that nonattendance a pure distinction between food products available for regular nourishing
- Foodstuff that does not attend the demanded nutritious purpose
- Foodstuffs that can damage human well-being
Conclusion
The FSSAI Rules for food supplement business have been modified, keeping in mind health issues and inferior supply of food supplement business in India. The food crops under this group can be shaped, factory-made, and wholesaled in the procedure of tablets, capsules, sauces, and more. In India, as a Food Business Operators, you can apply for Basic FSSAI License if your revenue is less than INR 12 Lakhs, FSSAI State license in case your income is More than INR 12 Lakhs and less than INR 20 Lakhs. CorpBiz shall be at your knock-head if you require any assistance on Food Supplement Business in India.
Read our article: Rules regarding mentioning best before & manufacturing date











