The Indian healthcare market is the fastest-growing market for medical products. Manufacturers worldwide are more inclined toward the Indian market due to the high potential for growth and long term profit. In India, medical devices are regulated by The Central Drugs Standard Control Organization. Being a statutory body, the CDSCO is held accountable for furnishing medical device registration to the business entities in India. The entities seeking medical device registration needs to fill up an application on the CDSCO SUGUM portal and upload the prescribed documents. In this blog, we will look into Documents required for Medical Device Registration in India.

Medical devices which seek registration are as follows:
- Cochlear implants
- Spinal needles
- Syringes and needles
- Annuloplasty rings
- Heart valve
- Endotracheal tubes
- Surgical sealants
- Orthopedic implant
- Catheters
- Cardiac stents
Read our article:What is the Importance of a Medical Device Certificate in India?
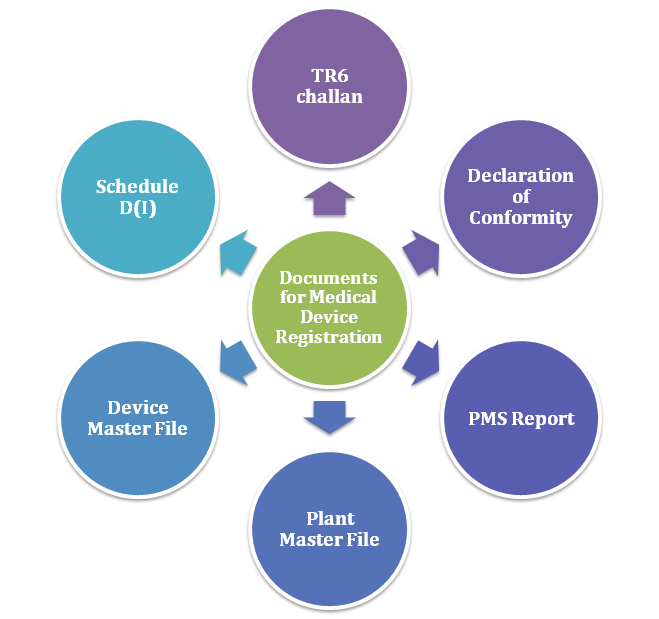
Documents Required for Medical Device Registration
Now let’s talk about the documentation part that you will need to upload on the CDSCO portal for the registration purpose.
Form 40
Form 40 is an application to obtain approval for product distribution in the Indian market. If your product falls under notifies product category, then you would need to get register under CDSCO via form 40.
TR6 Challan
TR 6 Challan is often utilized to address the payment of service tax. It can also be used to pay customs duty for the imported product.
Schedule D (I)
Schedule D(I) enclose the details and undertaking need to be filled by the manufacturer or agent via application form for a registration certificate. The format must be appropriately filled in for application in Form 40. The applicant should maintain the confidentiality of such information and must deliver the content in an encrypted format.
Schedule D(I) seeks the following particulars from the applicant willing to obtain a registration certificate.
- Details of the manufacturer and its manufacturing facilities.
- Details of the drug that is produced at the facility along with existing certificates.
- Undertaking to declaration
Declaration of conformity
A declaration of conformity is a sort of legal document that states a product (from the electronic category) meets the regulations laid down by the statutory body, such as CE, to assure safety.
Post-market surveillance (PMS) report
Post-market surveillance (PMS) report is a general assessment report enclosing past data that help to depict complaint trend for specific medical equipment. Such a report can help the manufacturers to overcome loose ends of products that are likely to overshadow its functionality in one way or another. It should be kept in mind that PMS is prepared against the existing products that were already sold and serving clients from different regions.
Plant master file
The plant master file is drafted by the manufacturer, and it encloses details regarding quality management policies, site activities, quality control, and operation. It should be kept in mind that the Plant master file should be site-specific, and it should contain information that exists in the plant. Thus, such a file should be confined around information regarding manufacturing practices and GMP related activities.
Free Sale Certificate
In India, the free sale certificate is issued by State licensing authority, and its purpose is to ease out the process of exporting the drug to the overseas country. A free sale certificate is a mandatory certification for medical device manufacturers who wish to cater to the global market. A free sale certificate gives the implications that the product is safe to use, and it is already available in the country of export.
Device master file
A device master file encloses the proprietary data regarding the component, material, or manufacturing process. The proprietary data doesn’t follow a prescribed format as it could vary accordingly as per the type of product or components. A typical device master file encloses test results, comparative analysis, toxicity test results, and biocompatibility[1] of the component and the material used in equipment.
ISO 13485 certificate
ISO 13485 is a globally recognized certification that serves the medical device industry. ISO 13485:2016 illustrates several requirements for a QMS that can be used by a manufacturer engaged with the stages of the life-cycle of the medical device. The standard also encompasses areas like design, development, manufacturing, storage, installation, servicing, and decommissioning. The supplier of medical devices can voluntarily opt for this standard to improve their market presence. The standard exhibits the manufacturer’s ability to meet the regulatory requirements consistently.
Power of Attorney
A power of attorney is a legal document that allows the property’s owner to transfer its legal power to another person, aka agent. The agent who obtains the POA could have the legal authority for decision making in relation to the principal’s property, finances, or medical care. A power of attorney is more likely to use in a condition when the property owner is not available for any reason to sign the legal document.
CE design certificate
CE design certification is usually availed by the manufacturers whose product falls under the EC/EU directives category. The medical product seeking CE mark needs to conform to the design requirement of such directives.
Other regulatory certificates
The manufacturer can club other product certifications furnished by the local authorities as well with the aforesaid documents. It could help the authority to speed up the registration process.
Illustrations on Documents Arrangements
Upon arranging aforesaid documents, head over to the CDSCO SUGUM portal and file the application in Form 8. Once you fill the application successfully, upload the softcopy of the aforementioned document on the portal. The authority usually takes a couple of months validates the submitted credentials. It should be noted that the registration certificate for medical devices remains valid for three years, and it should be renewed within six months before the expiration date. The overseas manufacturers who have FDA approval for their product can use such registration to leverage their product’s authenticity.
Conclusion
That’s all about the Documents required for Medical Device Registration in India. If you have a second thought on this topic, kindly let us know through the comment section. We would value your participation and try to overcome your doubt through professional advice. The CorpBiz is a highly valued destination for business entities and individuals looking to establish a new India startup. We help our clients obtain mandatory licenses to run the business and help them keep up with ever-evolving compliances.
Read our article:A Step by Step guide for Registration Process for Medical Devices in India