Previously, the medical devices manufacturers were free to sell their products in the Indian market without any regulation. Since 2006, the medical devices imported from overseas locations need to comply with Indian medical device regulations established by CDSCO. The CDSCO (Central Drugs Standard Control Organisation) is a statutory body that laid down rules for new drug and clinical trials. In this blog, we will focus on the Registration process for medical devices in India.
Classes of Medical Devices
The medical devices in India have been categorized into four classes based on the criticality and risk they offer to the patients.
- Class A: Represent the low-risk category of the products such as thermometers, and tongue depressors.
- Class B: Represent moderate-risk products such as suction equipment and hypodermic needles.
- Class C: Represent moderate-high risk products such as bone fixation and lung ventilator.
- Class D: Represent high-risk products such as implantable devices and heart valve.
Read our article:Four Prominent Benefits of Drug license that you must know
Guidelines for Registration Process for Medical Devices
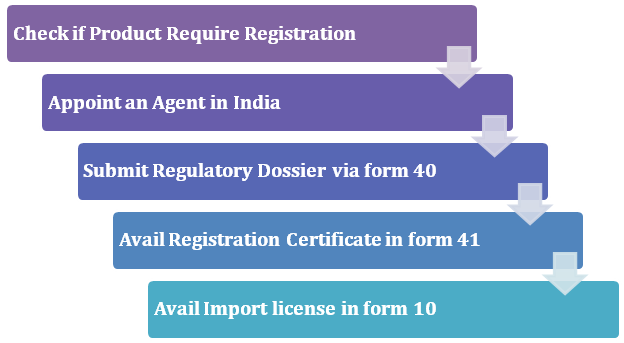

Check if Product Seek Registration
In India, the manufacturing and distribution of medical devices are regulated by the Drug and Cosmetics Act, 1947[1]. Presently, the act covers the 22 notified medical devices. The following section demonstrates the list of devices comes under the said act.
- Cochlear implant
- Spinal needles
- Tracheostomy tubes
- Cardiac stent
- Syringes
- Endotracheal tube
- Dental implant
- Annuloplasty ring
- Catheters
- Surgical sealants
The list is not over yet, as many more devices come under the influence of said act. In some circumstances, DCGI scrutinizes specific product details and could confer NOC regarding the medical devices’ exemption. The whole process takes one to three months to reach completion.
Hire Authorized Indian Agent
The registration cannot be granted to someone who not from the Indian territory. The said act allows the overseas manufacturers to hire an authorized Indian agent to address the registration formalities. The manufacturer needs to ensure that the hired professional should be registered under CDSCO. Plus, he/she should possess a whole drug license as well in the form of 20B and 21B. Once availed, the registration certificate will allow the manufacturer to appoint multiple distributors in India.
Submission of the Dossier under Form 40
This is the first and foremost step in the registration process. Here, the hired agent needs to prepare and submit a dossier against the requested list of documents. The following list demonstrates the type of documents required for registration purposes.
- Form 40
- TR6 Challan
- Schedule D(I)
- Power of attorney
- Quality assurance certificate
- Conformity declaration
- PMS* report
- Master file of the device
- Master file of the plant
- Sale certificate
- Regulatory approvals
- CE certificate
What does PMS stands for?
PMS – PMS stands for post-market surveillance. It is an accumulation of the compliant data received over the period of time. It helps the manufacturer depict trends in the complaint data to overcome the area of vulnerabilities within the equipment to ensure better functioning.
The registration fees charged by the CDSCO for a single manufacturing unit is US$1500. Furthermore, if the applicant seeks registration for a particular set of devices, then the registration fees would be US$ 1000. The authority might take anywhere between six to nine months to complete the process. The processing may be extended if the product doesn’t have a base in the Indian territory. In such cases, the authority appoints a special committee that scrutinizes the products on different grounds and their potency for the Indian market.
Avail Registration Certificate in Form 41
After investigating the submitted documents, CDSCO will respond to the authorized agents with a query letter. The agent needs to answer the queries and deliver them back to the authority within the requested timeframe. Upon receiving the answers, the authority shall test the applicant’s response and eventually decide to grant the license. In case if the authority is not satisfied by the response, it may forward another query letter to the applicant.
Avail Import License in Form 10
In order to avail of import license in form 10, an application has to be filed in Form 8 and 9 at the CDSCO SUGUM portal. The authority usually process such a request in 4 to 12 weeks.
Once the applicant avail the import license and registration certificate, the shipment of medical devices can enter the Indian territory. Furthermore, the manufacturers can sign a contract with distributors for selling their products across the Indian market. The registration would serve the validity period of three months.
Duties Serve by the Authorized Agents
- Provide constant feedback to the manufacturer regarding the policy changes or obnoxious events
- Connect with the authority to apply for a medical device certificate.
- Arrange mandatory from the manufacturer for the registration purpose.
- Coordinate with manufacturers to share post-market surveillance data and adversaries.
Minimum Eligibility Criteria for Authorized Agents
- Candidates should belong to the Indian territory and have few years of experience in the health care industry.
- Candidates should possess “Power of attorney” to submit documents related to medical device certificate at CDSCO.
- Candidate should have a drug wholesale license under the possession in the form of 20-B and 21-B.
Valuable Pointers regarding the Registration Process for Medical Devices
- CDSCO is the prominent regulatory body in India that lays the guidelines for medical devices and pharmaceuticals to ensure the patients’ safety and well-being. Central Drug Standards Control Organization (CDSCO) continually trying to impart transparency and uniformity in its system to ensure the quality of the medical products. CDSCO has synced various state regulators for granting the license for specific drugs, vaccines, and IV fluid.
- The Drug Controller General of India (DCGI) is one of the key representatives within CDSCO who is responsible for sanctioning the new drugs and medical devices in India.
- Drugs & Cosmetic Act and Rules (DCA) is a statutory framework that outlines the directives for the drug and medical equipment in India.
Conclusions
The registration of medical devices is crucial in India for distribution purposes. The concerned authority would penalize any manufacturer who wish to sell the product in unregistered condition. Connect with CorpBiz’s professional to avail professional help on drug licenses and other registration in India.
Read our article:What is the Importance of a Medical Device Certificate in India?