Medical devices in India are manufactured against the predetermined compliances to ensure product safety. Previously, the distributors, importer, and manufacturers of medical devices sold their products without any license. Unfortunately, such liberties have been laid off by the government a few years back to ensure the protection of human life. Now, every manufacturer who wants to dive into medical equipment and drug manufacturing needs to register from a regulatory body called CDSCO. There are thirty class of products that come under the radar of CDSCO and seek registration to get sold in the Indian market. In this blog, we will explain the importance of a Medical device certificate in India.

Importance of a Medical Device Certificate
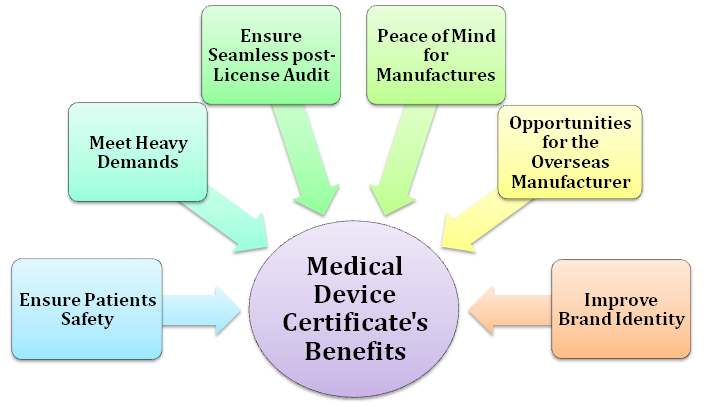
The importance of Medical Device certificate are stated below:-
Ensure Patients Safety
The ultimate goal of manufacturing medical devices is to aid patients. They should be free from vulnerabilities or harmful effects. Hospitals across the nation seek high-quality medical devices to render the best possible treatment to the patients. With the patient’s life at stake, the margin of error for these devices is next to negligible. It won’t’ be possible to judge the product quality by merely looking at it, and that skepticism could be dangerous for human life. The hospital needs to ensure that every device is used to help the patient be 100% operational and free from vulnerabilities. The product certified by the CDSCO promises to deliver uncompromised performance with exceptional service life and quality, depending on the class they represent. These products are supposed to be tested at the certified lab. The authority which tested these products for safety often takes months to approve them. Thus, with registered medical devices at the disposal, patients shouldn’t be concerned about such equipment’s legitimacy.
Meet Heavy Demands
India is a prominent marketplace for the manufacturer of medical devices coming from different part of the world. To meet heavy demands, the Indian government is heavily relying on overseas manufacturers. With so many products at disposal, the regulatory body is not taking chances with the quality of the product. That’s why the regulatory body has been set up to rectify the moderate and poor quality products.
Ensure seamless Post-License Audit
The authority generally conducts the on-site audit of the medical devices to ensure whether products are being manufactured and stored as per given compliances. With registration license at the disposal, the manufacturers can help the audit team to skip the unnecessary parts and focus on the key areas. Also, the audit helps to identify the potential faults in the equipment, helping to overcome vulnerability that might affect the patient in one way or another. It should be in mind that The Drugs and Cosmetics Act, 1940[1], encloses the provisions for severe penalties in case of the infringement of the given regulations that were found during the audit. So keeping up with the compliances must be the priority of every manufacturers and distributor.
Peace of Mind for Manufactures
Since it has been made mandatory to avail license to manufacture medical devices, the country’s manufacturers were under the relentless pressure of maintaining compliance with given law. However, those who are keeping up with compliances and directives need not worry a lot. All they need to prepare and distribute their product according to the prescribed guidelines and rest will be managed automatically.
Opportunities for the Overseas Manufacturer
The escalating demand for medical devices pushes the Indian government to open the gate for the overseas manufactures. As a result of that, CDSCO now allows overseas manufacturers to send their shipment of medical devices in the Indian market after complying with prescribed guidelines. Although the process availing the import license is a tedious one, it won’t bother the manufacturer until the profit margin remains on the higher side.
Improve brand identity
Previously, the manufacturer was not under the obligation to avail license to sell their product in the Indian market. Although this helped them focus on core competencies rather than compliances, it won’t cement their place in the market due to the absence of identity. With new norms and regulation in place, now every manufactures have opportunities to explore the market dynamically after getting their product registered under a given law. It doesn’t make sense to infringe the guideline for the sake of profit since the penalties are too steep to handle.
Read our article:A Step by Step guide for Registration Process for Medical Devices in India
CDSCO’s Role
CDSCO is a regulatory body that holds the CLA and SLA to grant permits to business entities seeking manufacturing and trading of medical devices in India. CLS cater to the requirement of
- Import device licensing
- Licensing regarding the Manufacturing and distribution of medical devices mainly from the category of class C and D.
On the other hand, SLA doesn’t grant licenses for importing the devices from the overseas location. They basically conferred license to the manufacturer and the distributors of medical of Class C and D devices. Authority can afford to take chances with the product’s quality aspect with so much demand in place.
Conclusion
There are plenty of reasons why manufactures should avail of medical device registration in India. With the government tightening medical devices’ compliance every passing day, it becomes important for the manufacturers to comply with the law regardless of their scenarios. The importance of a Medical device certificate is enormous if we focus on long-term benefits.
Having said that, if you need some help in availing drug licenses or medical device registration in India, then we can assist you in a professional way. All you need to connect with CorpBiz’s professional and rest will be handle by the experts.
Read our article:Four Prominent Benefits of Drug license that you must know