An organization that lacks substantial policies, follows the vague framework, and has unclear SOPs, often suffers from losses either in terms of finance or credibility. To address this concern, organizations often revamped the overall structure instead of addressing the pain point and that’s where ISO certification comes so handy. International Standardization of Organisation is an apex body that is best known for developing a quality management system for various organizations regardless of their size and type. It creates the roadmap for growth and long-term profit for the organization. In this blog, we will be going to unfold the Documents required for Global Quality Certification.

ISO Policies: Global Quality Certification
Deployment of ISO policies can help firms fix the system’s issues that are accountable for compromised efficiency and quality. To avail such a certification, the organization needs to arrange some mandatory documentation. But before we address the documentation part, we would like to draw your attention to the type of ISO registration.
- Quality Management (ISO 9001 2008)
- Environmental Management (ISO 14001)
- Information security Management (ISO 27001)
- Food Safety Management (ISO 22008)
As it is clear from the above table, for obtaining a standard quality certification, one must opt for the ISO 9001. The documentation part for the ISO 9001 is a bit tricky and seeks extra attention from the applicants who are willing to obtain such a certification.
Following are the grounds that might impact the documents required for ISO 9001 certification
- Size of the organization
- Type of activities performed within the organization
- Type of products and services rendered by the organization.
- Type of operations and functions performed within the organization.
- Competence of the management and employees to meet the requirement.
Read our article:Why the ISO Registration Has Creditability in India: Process of ISO Certification
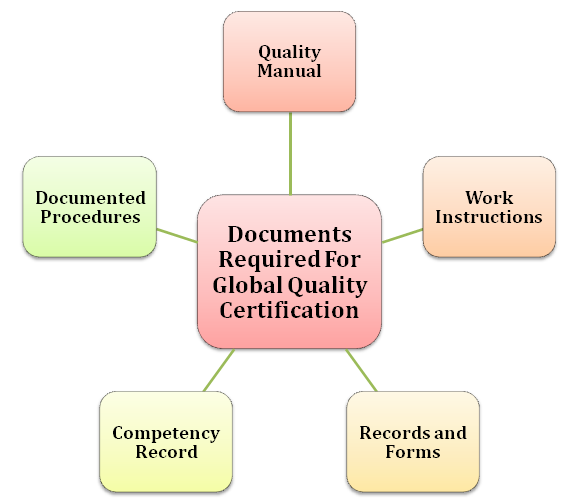
Documents Required for Global Quality Certification
The documentation process for ISO 9001 is categorized into four levels which are as follow:
Level 1 – Quality Manual
The very first document that an organization needs to deploy pertaining to ISO 9001 is the quality manual. Such a document encloses hierarchical information of the organization and the policies stating how the respective department will achieve the given objectives within the boundary of the quality management system. The quality manual also left scope for the inclusion of performance-sensitive goals that impact the organization’s decision-making[1] abilities in one way to another.
Level 2- Documented Procedures
Every organization must follow predefined working protocols that should be enclosed in a principal document. Such a document reflects all the procedures regarding the organization’s task against the relevant quality standards.
Level 3-Work Instructions
A work instruction is a document that reflects the information of the tasks carried out by the employees within the organization. It even encloses the minute tasks that have little impact on overall productivity or efficacy.
Level 4- Records and Forms
Such documents use to include information regarding the completed tasks. The management can gauge these documents later to depict the pain points that are accountable for inefficiency.
Competency record
Organizations must figure out the specific competencies for every employee. While addressing such a task, the management should look into various aspects of employee’s skills to meet particular job profile criteria. Such details should be adequately documented and then communicated to concerned departments. The competencies record is usually created based on the training, experience, skill, and qualification of the applicant.
As mentioned earlier, the number of documents might vary for different organizations depending on their size, processes, and other factors. Currently, ISO 9001 revolves around six procedures. However, in the case of quality processes, some companies might include new sets of guidelines. Following is the list of techniques defined by ISO:
- Control of Documents
- Control of Records
- Corrective Action
- Control of Nonconforming Product
- Preventive Action
- Internal Audit
That’s all about the Documents required for Global Quality Certification. Now let’s move on to the next section.
ISO 9001 Seeks the Given Forms
- Customer property
- Purchasing process
- Design and development verification
- Management Reviews
- Internal audit
- Identification and traceability
- Monitoring and measurement of the product
- Design and development output
- Control of production and service provision
- Competence, awareness, and training
- Design and development validation
- Design and development inputs
- Design and development changes
- Planning of product realization
- Control of monitoring
- Review of Design and development
- Design planning output
- Corrective action
- Review of requirements
- Validation of processes related to production and service
- Preventive action
- Control of nonconforming product
- Analysis of data
Why should you opt for ISO 9001 certification?
- Global Quality Certification improves the organization’s performance and efficacy.
- Create a roadmap for sustainable development.
- Render better control to management to optimize the existing process for maximizing the production.
- Global Quality Certification improves the current quality management system.
- Reduce shortcomings or vulnerabilities by overcoming errors within the circles.
- ensures seamless correspondence between different departments.
- Reduce inefficiency through continual improvement of the existing quality protocols.
- Global Quality Certification promotes transparency
- Overcome uncertainties by setting up a simple working framework.
Conclusion
ISO certified companies reflect that they are following a prescribed rule and relegation to ensure the quality of their product. Perfect documentation can ensure the error-free implementation of ISO 9001 standard within the system. Developing precise documentation for such a certification could a daunting undertaking for anyone. It’s because one has to arrange large pile documents required for Global Quality Certification. In such a case, we recommend you to hire an expert that can take care of the tedious process and assist you in deploying ISO standards within your system. The CorpBiz has an elite team of professionals who provide class-leading consultancy on various compliances and government-driven certification.
Read our article:An Overview on the Prominent Benefits of Global Quality Certification











