For conducting a cosmetic business in India, one has to comply with plenty of legal implications. One such requirement is obtaining cosmetic registration from Central Drug Standard Control Organization (CDSCO). At present, cosmetic registration comes under the Drugs & Cosmetics Act, 1940, which exclusively defines the term Cosmetic as an article used for the human skin. As per the Act, its primary utilization includes cleansing, beautifying, and promoting attractiveness.
What are the Types of Cosmetic Registration in India?
Cosmetic registration is required when an individual wishes to manufacture a product portfolio that deals with beautification or skin cleansing. Therefore, the following is the registration that is required for the manufacturing and distribution of cosmetic products in India as per the Drugs & Cosmetics Act, 1940.
- The license in Form no 32 is granted for manufacturing/sale of cosmetic products in India.
- The license in Form 32-A is granted for loan license for manufacturing cosmetic in India.
- The license in Form 37 is granted for the renewal or approval for conducting tests on cosmetic products or raw material used in the manufacturing thereof on behalf of the manufacturing license for sale of drugs and cosmetics (Form no. 36).
- License in form 42 for the importation of cosmetics from the other nations.
Who Regulates the Cosmetic Registration in India?
Central Drug Cosmetic Registration in India Standard Control Organization (CDSCO) is the one who looks into the regulatory aspects of Cosmetic Registration in India. All the prerequisites related to cosmetic registration are required to be regulated by this authority. The law which oversees the cosmetic business is the Drug and Cosmetic Act 1940 & Rules 1945.
Apart from this regulatory body, the BIS introduce many standards for cosmetic registration in India. These bodies are also accountable for rendering the proportionality of the ingredients used in such products.
What are the Guidelines for Manufacturing and Importation of Cosmetics in India?
The following section depicts the general guidelines related to the manufacturing and the importation of cosmetics in India.
Signing Undertaking Stating- No Animal is harmed During the Testing of the Product
It is clearly cited that any importer of the cosmetic product prior to the registration of cosmetic registration must furnish an undertaking reflecting that the product has not been tested on the animals. In addition to this undertaking, the importer also has to submit import registration details to the CDSCO. Upon receiving the said dossiers, the authority will furnish the acknowledgment slip, which can be used for future referencing.
Letter of Authorization if Third-Party is Manufacturing outside India
In case when the brand owner is outside India then the manufacturer requires signing a letter of authorization to brand owner & authorize him to do an act on behalf of the owner which authorize the importer to perform required duties in the behalf of the manufacturer residing outside India & this letter is considered as Power of Attorney.
Import Cosmetics in Bulk
The applicant can avail a certificate related to the free sale and bulk importer for sale of the same products can obtain customs clearance approval after the cosmetic business license testing.
What are the Cosmetic Regulations for Cosmetic License in India?
Under the Drugs and Cosmetic Rules, 1945, Schedule M-II bifurcates cosmetics into 11 extensive products categories:-
- Powders
- Creams, lotions, cleansing, milk, shampoos, pomade, shaving creams, hair oils etc.
- Nail polishes and Nail lacquers
- Lipsticks and Lipgloss
- Depilatories
- Preparations used for eyes
- Aerosol
- Alcoholic Fragrance Solutions
- Hair Dyes
- Tooth powder and toothpaste
- Toilet soaps
To manufacture any product from the above list, registration is required to be availed from the concerned authority designated by the State government. The application has to be furnished in Form 31 along with a prescribed fee of Rs 2500 and, with an inspection, a fee amounting to Rs 1000.
Now before granting the registration, the authority shall conduct an in-depth inspection of the whole premises. Also, the inspector is to be designated as per the Act. The inspection officer requires submitting the comprehensive report to the authority, which will decide whether to furnish the license or not
Qualification Criteria for Staff Working in Cosmetic Manufacturing Unit
The manufacturer needs to ensure that the hired staff is qualified enough to carries out production activity. The employed personal should comply with the following qualification criteria.
- He/she must possess a diploma in pharmacy from a certified institution that operates under the Pharmacy Council of India governed by Pharmacy Act, 1948, or
- He/she must register under the Pharmacy Act, 1948 or
- He/she must pass the 12th standard with chemistry or any education as the authority may deem fit.
Regulation for Manufacturer Concerning Labeling of Products
The list below contains the labeling requirements as per the Drugs and Cosmetic Rules, 1945:
- The product’s name ought to be clearly mentioned on the inner as well as the outer labels, and in case the container is not big in size, then the principal place & the pin code are fine.
- The outer label must reflect the ingredients that are used in the product’s manufacturing.
- The inner label should enclose the direction of use and necessary warnings to ensure safety.
- A separate manufacturing batch preceded by letter with “M” and batch number “B” must be mentioned on the label.
- The Bureau of Indian Standards (BIS) performs quality check of these products from time to time and makes necessary changes in the Indian standard.
What Documents are required to Avail the Cosmetic Registration in India?
- Original copy of Power of Attorney reflecting the legal connection between the importer and manufacturer.
- Certificate of free sale, marketing authorization letter
- Form 42 duly filed and signed
- Schedule D III duly signed by the manufacturer or his/her authorized agent.
- Free sale certificate/Market authorization letter/Manufacturing license if any.
- Product specification & testing protocols.
- NOC from Pollution Control Board
- Plan the layout of the premises
- Rent agreement in case of rented premises
- List of laboratory equipment
- List of machines installed for manufacturing
The manufacturing and importation of cosmetic products should be done in accordance with the Drugs and Cosmetics Rules, 1945. The cosmetic registration remains valid for three years.
Online Process for Registering Cosmetic in India
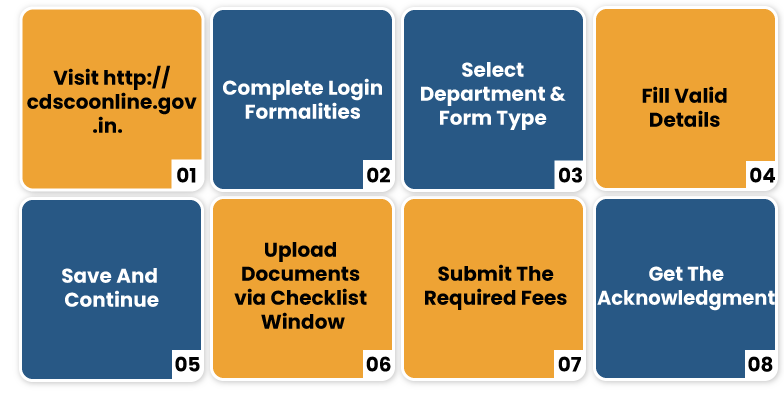

- Visit http://cdscoonline.gov.in. and complete the login formalities or create a new one
- Under submission section form, select department & form type
- Select the required form and fill it with valid details
- Click on the “Save and Continue” button to proceed further
- On the Checklist Window, upload the requested documents one by one.
- Click on the “Submit” button to proceed further. The payment page will appears
- Submit the required fees and tap on the “PDF” button to get the acknowledgment slip.
Conclusion
The Drugs and Cosmetics Act, 1940[1] entails the provision regarding the violation of Act under which a defaulter can confront imprisonment up to one year and a fine up to INR 1000 or both.
In case of multiple convictions, the imprisonment period and fine amount can be extended proportionately. Therefore, it is essential to ensure conformity with the norms under the said Act. You can obtain cosmetic registration without hectic paperwork and tedious process if you opt to avail the CorpBiz’s services.
Read our article:Import License of Drugs and Cosmetics in India











