Wish to ship export food items from India to the USA? Sounds like a profitable business option. Welcome to the exciting regime of foreign trade, i.e. imports and exports. As per the Foreign Trade Policy of Indian, the exporter should avail an Import Export Code (IEC) before commencing the EXIM business. Import Export Code is nothing but a 10-digit numerical code bestowed by the Directorate General of Foreign Trade (DGFT) to any Indian entity that seeks to do EXIM business from India. Getting certified by FDA is another imperative requisite for shipping food items to the United States. It is an offence to sell food items in the United States without FDA registration. Apart from IEC and FDA registration, the exporters are also required to other mandatory registrations which we have discussed in the latter part of this blog.
Who are liable to register under FDA?
- US-based Facilities or entities belong to the food sector
- Facilities from overseas nations willing to ship food items to the United States. Such facilities are mandated to register under FDA via a registered agent.
How to Get FDA Registration from the USA?
Step 1: Appoint a licensed Agent, aka FDA agent.
You must appoint a licensed agent before engaging with the registration process for your business. The FDA agent will act as a bridge between FDA and a foreign establishment like you. He will also help the authority in planning mandatory inspection & address all the concerns on your behalf raised by FDA.
You need to share the agent information with authority via FURLS (FDA Unified Registration and Listing System).
STEP 2: Furnish FDA registration application form
All overseas & local food businesses must furnish a registration application on FDA Unified Registration and Listing System.
STEP 3: Grant of a license number by FDA
After completion of the registration process, the authority will grant an 11-digit registration number. The information regarding the same will also be shared on your registered email id.
STEP 4: Grant of the FDA registration
You will get the certification of registration from your appointed agent. The registration information will not be shared with third-party by the US-FDA.
An overview on the Renewal of FDA registration
W.e.f 2011, the FSMA mandates the renewal of FDA registration to ensure compliance of norms mentioned by USFDA. All facilities involved with the packing, processing, or storing foods, beverages & nutraceuticals consumed in the USA have to renew their FDA registration. The renewal requires the authorization of FDA agents.
It is essential to note down that the US FDA can cancel the registration if it is not renewed before the predetermined date (which is set to 31st Dec), in a biennial period. Under these circumstances, all the food items shipments would be contained at the US port and will be released only after proper renewal.
Online Process to obtain Import Export License from DGFT
IEC registration process is divided into the five important sections as shown as
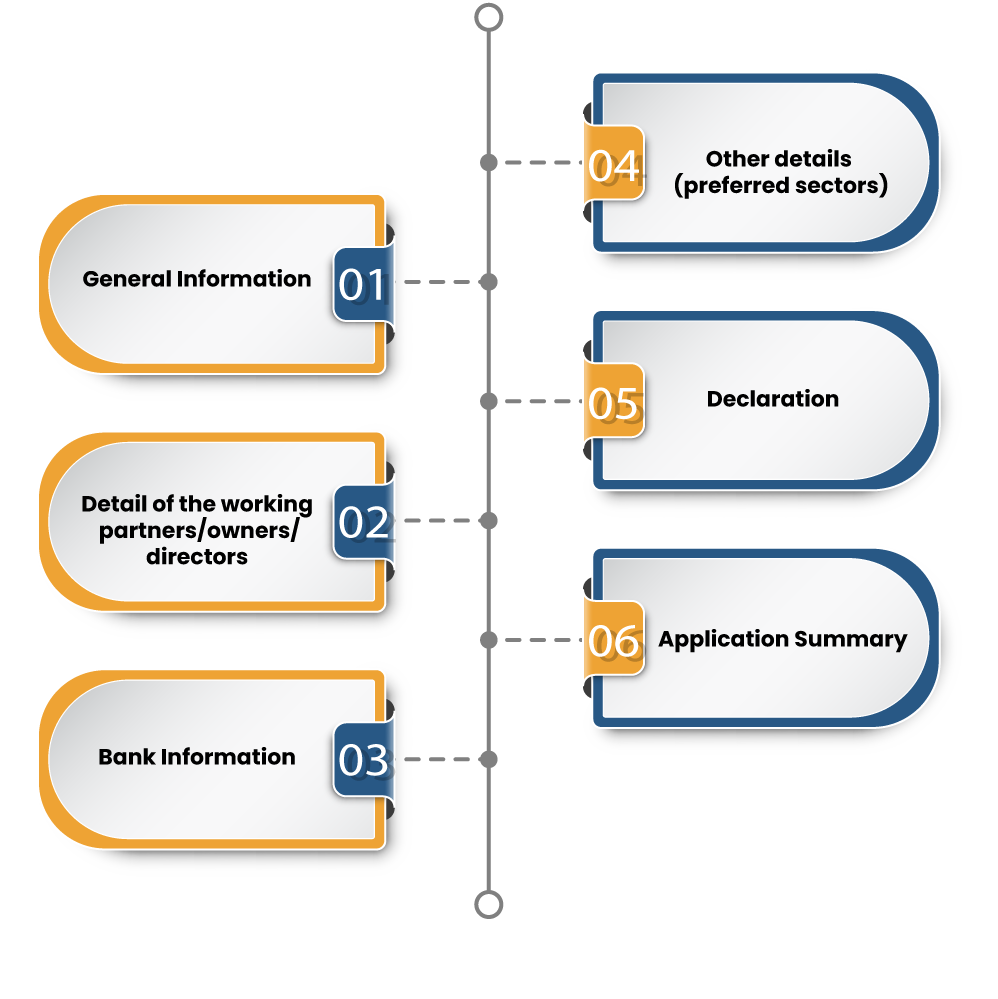

Here are the online instructions to avail of the IEC certification from the DFGT portal
- Open the DGFT web portal and log in to the same.
- Next, tap on the option: ‘Apply for IEC’. This will land you to on the next page.
- Next, tap on “Start Fresh Application”. This action will land on the e-form, where you will be prompted to provide the following details
- Nature of Firm
- Firm Name
- PAN number
- Date of Incorporation
- Category of Exporters
- Corporate Identity Number
- GSTIN Number
- Firm Mobile Number
- Email Address
- On the same window, upload the proof of establishment/registration/ incorporation for the given entity in the prescribed size and format.
- Scroll down to the next section, i.e. Firm Address Details to enter the address of your business premises along with the “Jurisdictional DGFT RA.”
- On the same window, upload the supporting documents such as a Sale deed, utility bill, Voter Id, Aadhaar number, NOC of the firm.
- Scroll down to the next section to enter the branch detail, if available.
- Once done, tap on the Save and Enter button to proceed to the next section, where you need to provide the detail of the management based on your business form. Also, you need to upload the supporting document in the prescribed format.
- Then, enter the bank details such as IFSC code, Branch name, Bank name, Account Name. Once you provide the said details, upload the copy of the cancelled cheque book and click on the “Save and Next” button.
- On the following section, opt for the Preferred sectors of operations followed by the Acceptance of Undertaking/Declaration. Next, click on the “Save and Next” button
- Make a payment on the next section either via DSC or Aadhar OTP.
Read our article:An Overview on Surrender of IEC License
Consent from Export Inspection council
The Export Inspection Council is an export–certification body that has laid down certain norms for the quality and safety of products shipped from India. The Indian government founded EIC u/s 3 of the Export (Quality Control and Inspection) Act, 1963, to ensure the seamless development of export trade of India via inspection & quality control and matters connected therewith. The main objective of this government-backed institution is to ensure that items cited under the Export (Quality Control and Inspection) Act 1963 adhere to norms of the importing nations w.r.t quality and safety.
EIC facilitates mandatory certification for a different types of food items such as dairy products, fishery products, egg products, honey, meat, animal casing, Ossein, Gelatine, and feed additive and pre-mixtures.
APEDA certification for the export of the Schedule products
Agricultural & Processed Food Products Export Development Authority, commonly known as APEDA, was established by GOI in 1985 via an act for the development & incentivization of export of scheduled products.
It facilitates fiscal aid, information, norms towards the development of scheduled items. The items cited under the APEDA act are known as schedule products, and exporters of such products are mandated to avail of APEDA Registration. Connect with CorpBiz to avail services for the same.
Conclusion
The USA has remained a prime importer of Indian commodities for years. It is hard for any Indian exporters to neglect the US[1] market since it adheres to the immense growth potential. If you are in the food sector and willing to extend your footprint beyond national boundaries, then the US market is something that you cannot overlook. The legalities mentioned above are mandatory by all means, particularly for those who seek to export food items to the US market. Connect with CorpBiz’s professional in case you have some doubts regarding the same.
Read our article:Step By Step Process of FDA Certification











