Drugs and cosmetics fall among the list of substances which must be delicately handled. While it can be a life-saving remedy, it can also be banned for inappropriate usage. This prompts needs for a regulation, which in India is regulated by the Drugs and Cosmetics Act of 1940. This article explores the norms pertaining to the drugs distribution, manufacture, and sale under the Drugs and Cosmetics Act 1940.
Restrictions on Manufacturing, Sale and Drug Distribution
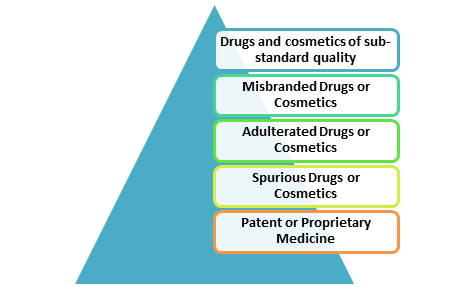
As per the directions of the Drugs and Cosmetics Act, the following drugs are prohibited from being manufactured for sale or from being offered for sale or for drug distribution:

Standard Quality of Drugs and Cosmetics
Standard Quality of drugs means that drug which complies with the standards set out in the Second Schedule, and that cosmetics which complies with such standards as prescribed in the same schedule. The Central Government, after consultation with the Drugs Technical Advisory Board, can amend the second schedule. The government after giving three months of notice of its intention to do so can amend accordingly by making notification in the Official Gazette.
Following are the Restricted Drugs and Cosmetics
Misbranded Drugs and Misbranded Cosmetics
A Drug is deemed to be misbranded as mentioned under Section-17 if it is colored, coated, powdered, or polished to conceal the damage.
The drug is also said to be misbranded if:
- It is made to appear of better or greater therapeutic value than it really is or if it is not labeled in the prescribed manner
- Its label or container or anything accompanying the drug bears any statement, design or device which makes any false claim for the drug or which is false or misleading in particular
The Misbranded Cosmetics as mentioned under Section 17-C is said to be misbranded if:
- It contains a color which is not prescribed or if it is not labeled in the prescribed manner
- The label or container or anything accompanying the cosmetic bears any statement which is false or misleading in particular
Adulterated Drugs and Adulterated Cosmetics
The Adulterated Drugs as mentioned under Section 17-A and The Adulterated Cosmetics mentioned under Section 17-E is said to be adulterated if:
- It consists of whole or in part, any filthy, putrid or decomposed substance.
- It has been prepared, packed or stored under unsanitary conditions whereby it may have been contaminated with filth or whereby it may have been rendered injurious to health
- Its container is composed, in whole or in part, of any poisonous or deleterious substance which may render the contents injurious to health
- It bears or contains, for purposes of coloring only, a color other than one which is prescribed
- It contains any harmful or toxic substance which may render it injurious to health
- Any substance has been mixed therewith so as to reduce its quality or strength
Spurious Drugs and Spurious Cosmetics
The Spurious Drugs as mentioned under Section 17-B and The Spurious Cosmetics as mentioned under Section 17-D is said to be spurious if:
- It is manufactured under a name which belongs to another drug or cosmetic
- It is an imitation of, or is a substitute for; another drug or cosmetics resemble another drug or cosmetic in a manner likely to deceive. If it is conspicuously marked such that it fails to reveal its true character or its lack of identity with such other drug or cosmetic.
- The label or container bears the name of an individual or company purporting to be the manufacturer of the drug, which individual or company is fictitious or does not exist
- It has been substituted wholly or in part by another drug or cosmetic
- It purports to be the product of a manufacturer of whom it is not truly a product
Prohibition of Drug Manufacture for Sale or Drug Distribution
Under Section 18, the person must not by himself or by any other person on his behalf manufactures for sale or for drug distribution, or sell, or stock or exhibit or offer for sale, or distributes few prohibited drugs or cosmetics.
Those are as follows:-
- Any drug which is not of a standard quality, or is misbranded, adulterated or spurious
- Any cosmetic which is not of a standard quality or is misbranded or spurious
- Any patent or proprietary medicine, unless there is displayed in the prescribed manner on the label or container thereof
- Any drug which purports or claims to prevent, cure or mitigate any disease or ailment
- Any drug or cosmetic which is manufactured in contravention of the conditions specified
- Cosmetics that consist of any ingredient which may render it unsafe or harmful for use
- Trading of any drug or cosmetic by not complying with the provisions of this Act
- Sell or stock or exhibit or offer for sale, or distribute any drug or cosmetic which has been imported or manufactured in contravention of any of the provisions of this Act
- Manufacture for sale or for drug distribution, any drug or cosmetic, except in accordance with the conditions of, a Manufacture drug license issued for such purpose under this Act.
The prohibition has an exception under the following circumstances:
- This provision does not apply to the manufacture of small quantities of any drug for the purpose of examination, test or analysis, subject to prescribed conditions.
- The Central Government after consultation with the Drugs Technical Advisory Board can permit to manufacture for sale or for distribution, sale, stocking, or exhibiting or offering for sale or drug distribution not being of standard quality, subject to prescribed conditions.
Manufacturer
Under Section 18-A, the person if so required is liable to disclose the name, address, and other particulars to Inspector of that person from whom he has acquired the drug or cosmetics. Exception is given to the licensed manufacturer of a drug or cosmetic or his agent for the drug distribution thereof in this provision.
Persons bound to disclose Place where Drugs or Cosmetics are manufactured
Under Section 24, the person for the time being in charge of any premises wherein any drug or cosmetic is being manufactured for sale or for kept for drug distribution must inform to inspector. He is also legally bound to disclose any other information to the Inspector about the place where the drug or cosmetic is being manufactured or is kept, as the case may be.
Read our article:How one can apply for Wholesale Drug License in India?
Maintenance of Records and furnishing of information
Under Section 18-B, the person holding a license of drugs and cosmetics must keep and maintain such records, registers, and other documents regarding drugs and cosmetics or as may be prescribed. He must furnish such information to any officer or authority exercising any power or discharging any function under this Act as is required by such officer or authority for carrying out the purposes of this Act.
| PENALTY | IMPRISONMENT | FINE |
| Penalty given Under Section 28 for Non-Disclosure of the name of the manufacturer | Whosoever contravenes with the provisions of Section 18-A or Section 24 must be punishable with imprisonment which may extend to one year | With fine of not less than twenty thousand rupees. |
| Penalty given Under Section 28- A for not Keeping Documents and for Non-Disclosure of Information | Whosoever contravenes with the provisions of Section 18-B must be punishable with imprisonment which may extend to one year | With fine of not less than twenty thousand rupees. |
Penalty for Manufacture, Sale, or Drug Distribution in Contravention to Act
- Any drug which is deemed to be adulterated under section 17-A or spurious under section 17-B or when drug being adulterated or spurious or not of standard quality is used by any other person causes his death or grievous hurt, then he must be punishable with imprisonment for a term which shall not be less than ten years andcan extend to a term for life. He must also be liable for the fine which must not be less than ten lakh rupees or three times value of the drugs confiscated, whichever is more.
- The person convicted under this clause must by way of compensation payable to the person who had used the adulterated or spurious drugs referred to in this clause
- If the person dies after the use of the adulterated or spurious drugs referred to in this clause then the person convicted must pay to the relative of the dead person who had died due to the use of the adulterated or spurious drugs.
- Any drug which is deemed to be adulterated under section 17A, other than a drug referred to in clause (a) used without a valid license as required under this Act must be punishable with imprisonment for a term which must not be less than three years and can extend to five years. He shall be liable for with fine which must not be less than one lakh rupees or three times the value of the drugs confiscated, whichever is more.
- The Court may after mentioning any adequate and special reasons in the judgment can impose a sentence of imprisonment for a term of less than three years and of fine of less than one lakh rupees
- Any drug which is deemed to be spurious under section 17-B, other than a drug referred to in clause (a) used must be punishable with imprisonment for a term which must not be less than seven years and can extend to imprisonment for life. He must be liable for fine which must not be less than three lakh rupees or three times the value of the drugs confiscated, whichever is more.
- The Court may after mentioning any adequate and special reasons in the judgment can impose a sentence of imprisonment for a term of less than seven years but not less than three years and of fine of less than three lakh rupees
- Any drug, other than a drug referred to in clause (a) or clause (b) or clause (c), in contravention of any other provision of this Act or any rule, made thereunder, must be punishable with imprisonment for a term which must not be less than one year and can extend to two years. He must be liable for fine which must not be less than twenty thousand rupees
- The Court may after mentioning any adequate and special reasons in the judgment can impose a sentence of imprisonment for a term of less than one year.
Penalty for Manufacture, Sale, or Distribution of Cosmetics in Contravention to Act
Under Section 27-A, whosoever himself or by any other person on his behalf manufacturers for sale or for drug distribution, or sells, or stocks or exhibits or offers for sale may be penalized under following circumstances:
- Any cosmetic which is deemed to be spurious under section 17-D or adulterated under section 17-E must be punishable with imprisonment for a term, which can extend to three years and with fine which must not be less than fifty thousand rupees or three times the value of the cosmetics confiscated, whichever is more
- Any cosmetic other than a cosmetic referred above if in contravention of any provisions of this Act or any rule made thereunder must be punishable with imprisonment for a term which can extend to one year or with fine which can extend to twenty thousand rupees, or with both.
Conclusion
License for drug distribution, manufacture, and sale requires conditions to be fulfilled as Licensee must have adequate storage facility, and applicant must be reputable in the occupation. The drug license granted for any of above must not be suspended or canceled unless the licensee has not been convicted any offense under the Drugs and Cosmetics Act 1940[1].
Read our article: How Retail Drug License essential for Pharmacy Business?