This year was the most important year for the medical device companies from the perspective of the medical devices and the regulations since the Medical Device Rules, 2017 has came into force on January 01, 2018. The Government has planned to amend the regulations for medical devices to considerably expand the medical device regulations reach. As the medical device sector remained unaffected by the outbreak of COVID-19 pandemic, the regulators have provided relaxations in the regulatory compliance as could be sufficient. India’s pharmaceutical and medical device regulator the Central Drugs Standard Control Organization (CDSCO) is a body under Directorate General of Health Services, Ministry of Health & Family Welfare. CDSCO is the National Regulatory Authority (NRA) of India and it has issued an extension for numerous types of different medical devices to register for getting the authorization in market. In this article, we shall deal with some of the important developments in the medical devices and regarding Extension of medical device compliance deadlines to 2021.
The CDSCO Guidelines
Effective from April 1, 2020 it shall be obligatory for the medical devices to get itself registered in India. Before the amendment, there were only 37 categories of medical devices were regulated but from 1st April 2020 all the medical devices have to oblige with the registration. The applicability for the extension of medical devices is particularly for all the importers and manufacturers of medical devices.
Read our article:Regulation for all Medical Devices under CDSCO Directive
Government Notification
The Government of India[1] issued two notifications on February 11, 2020, i.e. The Medical Devices (Amendment) Rules, 2020 and a new definition of the medical devices. The collective consequence of these two notifications is that all the medical devices shall be brought under the cover of safety and quality regulation from the date April 1, 2020 effective for both notifications.
The CDSCO extension of medical devices signifies that the medical device types that were originally expected to register itself under India’s Medical Device Rules, 2017 by the time period of January 1, 2020, can now register with the extension of medical devices until January 2021. CDSCO’s expansion of the definition and extension of the medical device aligns with the Global Harmonization Task Force (GHTF), which is efficiently increasing the extent of products that shall require registration as devices with the purpose of being sold in India.
Notification to Regulate All Medical Devices
According to the notification that is effective from April 1, 2020, all the medical devices that fall under the definition given below shall be regulated as “drug” under the MDR and DCA.
All devices including an apparatus, instrument, implant, appliance material or any other article used in combination or alone, with a software or an accessory, and the manufacturer intended to use it specially for animals and human beings or that does not accomplish the prime intended action on animals or human body by any immunological, pharmacological or metabolic means, but which may help in its intended purpose by such means or for more than one of the specific purposes of:-
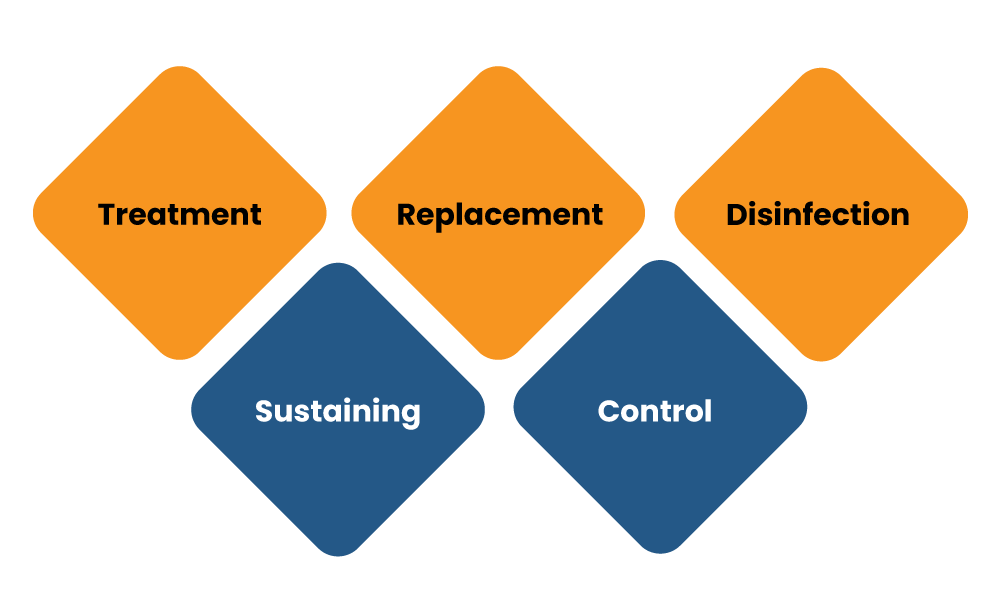

- Treatment, diagnosis, monitoring, prevention or alleviation of any disorder or disease, assistance for any kind of disability or injury;
- Replacement, modification, investigation, or support of a physiological process or of the anatomy or;
- disinfection of medical devices;
- Sustaining or supporting life; and
- Control of conception.
The new definition mentioned above is intended to cover up all the medical devices under the extension of medical device. Thus, because of this definition all the medical devices getting sold in India shall be regulated from April 1, 2020, after the taking effect of the definition.
The Rules states that these medical devices ought to be registered with the Central Licensing Authority by an online identified portal as has been established by the Central Drugs Standard Control Organisation (CDSCO). The registration will be voluntary for a time period of 18 months, after that it shall be mandatory.
The Complete List of Notified Devices
- Disposable Hypodermic Syringes
- Disposable Hypodermic Needles
- Disposable Perfusion Sets
- IVD Devices for HIV, HBsAg, HCV
- Cardiac Stents
- Drug Eluting Stents
- Catheters
- Intra Ocular Lenses
- Cannulae
- Bone Cements
- Heart Valves
- Scalp Vein Set
- Orthopaedic Implants
- Internal Prosthetic Replacements
- Blood Grouping Sera
- Ligatures, Sutures and Staplers
- Intra Uterine Devices (CuT)
- Condoms
- Tubal Rings
- Surgical Dressings
- Umbilical tapes
- Blood/Blood Component Bags
- Ablation Devices
- Organ Preservation Solution
- Blood Pressure Monitors (Effective January 1st, 2021)
- Digital Thermometers (Effective January 1st, 2021)
- Glucometers (Effective January 1st, 2021)
- Nebulizers (Effective January 1st, 2021)
- X-Ray Machines (April 1st, 2021)
- CT Scan Equipment (April 1st, 2021)
- MRI Equipment (April 1st, 2021)
- PET Equipment (April 1st, 2021)
- Defibrillators (April 1st, 2021)
- Dialysis Machines (April 1st, 2021)
- Bone Marrow Cell Separators (April 1st, 2021)
- All Implantable Medical Devices (April 1st, 2021)
- Ultrasound Devices (November 1st, 2021)
- Disinfectants and insecticides as specified in Medical Device Rules, 2017
New Registration for the Manufacturers
- Under Chapter IIIA: Registration of Certain Medical Devices of the Medical Device Rules, 2017 – the CDSCO has added a new section which sets the new registration for the manufacturers wherein the required certifications and documentation shall be uploaded for regulatory reviewing through an online portal.
- The medical devices at present notified under the Medical Device Rules, 2017 will not meet the criteria for the Chapter IIIA registration option. It suggests that new route could be an option for the products that shall be notified in April under the amendment extension of medical device in India. The devices that are entered into market via this expedited route will be exempted from the other requirements of the Medical Device Rules, 2017.
- The registration under Chapter IIIA shall be voluntary for 18 months abiding with the extension of medical device route implementation.
Conclusion
Any violation of Medical Devices Rules that also includes the failure to obtain license or registration before the fixed deadline shall result in to criminal prosecution which can either be imprisonment or fine or both. Also such stocks of medical device sold without license or registration could be confiscated.
Read our article:A Step by Step guide for Registration Process for Medical Devices in India