The term “drug” as defined in the Act includes a wide variety of substance, diagnostic, and medical devices. The act defines “cosmetic” as any product that is meant to be applied to the human body for the purpose of beautifying or cleansing. In 1964, the act was amended to include Ayurveda, Siddha, and Unani (ASU) drugs.
Ayurvedic, Siddha and Unani drugs include all medicines intended for internal or external use for or in the diagnosis, treatment, mitigation or prevention of disease or disorder in human beings or animals, and manufactured exclusively in accordance with the formulae. The provision related to the manufacture and control of Ayurvedic, Siddha, and Unani drugs have been prescribed in the Drugs and Cosmetics Act, 1940. The individual section being described here is based on the regulation as prescribed in the Drugs and Cosmetics Act 1940[1], for the Ayurvedic, Siddha and Unani hereinafter ASU drugs.
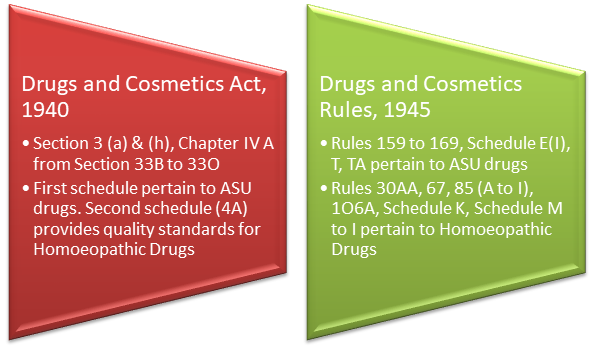

Provisions as amended under Drugs and Cosmetics Act
Central Government has the powers to frame and amend the regulatory provisions and issue a direction to the State Governments for their enforcement. In this regard, the Central Government is advised by the Ayurveda, Siddha, Unani Drugs Technical Advisory Board (ASUDTAB) and Ayurveda, Siddha, Unani Drugs Consultative Committee (ASUDCC), which are statutory bodies under the provisions of Drugs & Cosmetics Act, 1940, in the regulatory matters and enforcement issues respectively pertaining to Ayurvedic medicines.
|
Sections |
Provision related to ASU drugs
|
|
Ayurvedic, Siddha and Unani Drugs Technical Advisory Board (ASUDTAB) Section 33-C.
|
|
|
The Ayurvedic, Siddha and Unani Drugs Consultative Committee (ASUDCC) Section 33-D. |
|
|
Misbranded drugs Section 33- E. |
ASU drugs are deemed to be misbranded:
|
|
Adulterated drugs Section 33-EE. |
ASU drugs are deemed to be adulterated:
|
|
Spurious drugs Section 33-EEA.
|
ASU drugs are deemed to be spurious:
|
|
Regulation of manufacture for sale of ASU drugs. Section 33-EEB. |
The person must not manufacture for sale or distribution, any ASU drug except in accordance with such standards, if any, as may be prescribed in relation to that drug.
|
|
Prohibition of manufacture and sale of certain ASU drugs. Section 33-EEC. |
The person either by himself or by other person on this behalf
|
|
Power of Central Government to prohibit the manufacture of ASU drugs in public interest. Section 33-EED. |
Central govt., in the public interest, if it is necessary or expedient so to do then, by notification in the Official Gazette, can prohibit manufacture of ASU drugs, after satisfied on the basis of any evidence or other material available before it that the
|
|
Government Analysts. Section 33-F. |
Central or State government or a state government may, by notification in the official Gazette, can appoint any person, with the prescribed qualification and does not have any financial interest in manufacturing and sale of ASU drugs. |
|
Inspectors. Section 33-G. |
Central or State governments can appoint any person with the prescribed qualification and do not have any financial interest in manufacture or sale of ASU drugs. |
|
Penalty for manufacture, sale, etc., of ASU drug in contravention of this Chapter. Section 33-I. |
Manufactures for sale or for distribution of any ASU drugs deemed to be
|
|
Penalty for subsequent offenses. Section 33-J. |
Whoever having convicted of an offense under 33-I must be punishable with imprisonment for a term not less than two years but which may extend to six years and with fine not less than Rs 5000. (vary as per 33-I section, subsections and clause) |
|
Confiscation-Section 33K. |
Any person convicted under the act, the respective stock of ASU drug can be confiscated. |
|
Application of provisions to Govt. departments Section 33-L. |
The provision in this chapter except 33-K must apply in relation to manufacture for sale, sale, or distribution of any ASU drugs by any department of government as they apply in relation to the manufacture for sale, sale, or distribution of such drug by any other person. |
|
Cognizance of offenses. Section 33M. |
|
|
Power of Central Government to make rules. Section 33N |
The Central Government may –after consultation with, or on the recommendation of, the board( ASUDTAB)- and after previous publication by notification in the Official Gazette, make rules for the purpose of giving effect to the provisions of this Chapter: In certain circumstances, Govt. may formulate rules but the board should be consulted within six months.
|
|
Power to amend First schedule Section 33-O |
The central Govt. after consultation with the Board and after giving, by notification in the official gazette, not less than three months’ notice of its intention so to do, may, by a notification, add to or otherwise amend the first schedule. |
Read our article:How to get a Manufacturing License of Drugs in India?
Provisions as under Drugs and Cosmetic Rules 1945
The regulatory framework for Ayurvedic medicines is by and large modeled on the lines for allopathic medicines. Drugs & Cosmetics Act, 1940 and Rules thereunder have exclusive provisions for regulation and quality control of Ayurvedic medicines. Regulatory Officers are appointed in the Central and State Governments to oversee the enforcement of legal provisions for Ayurvedic medicines.
Manufacturing of Ayurvedic medicines need Drug license from the concerned State Government as well as compliance to Good Manufacturing Practices (GMP) and the standards prescribed in the Ayurvedic Pharmacopoeia. Proof of safety and effectiveness is required for licensing of various categories of Ayurvedic medicines.
|
Rules |
Provision related to ASU drugs |
|
Manufacture on more than one set of premises Rule 151 |
Separate license, for each premises, is to be obtained for manufacture of ASU drugs at more than one premises |
|
Licensing authorities Rule 152 |
The State Govt. shall appoint licensing authorities by notification in official gazette |
|
Application for license to manufacture ASU drugs Rule 153 |
An application for the grant or renewal of a license to manufacture for sale any ASU drugs shall be made in Form 24-D to the Licensing Authority along with a fee of rupees one thousand. |
|
Application for Loan Licence. Rule 153-A. |
An application for the grant or renewal of a loan license to manufacture for sale of any ASU drugs must be made in Form 25-E to the Licensing Authority along with a fee of rupees six hundred. |
|
Form of license to manufacture ASU drugs: Rule 154. |
Subject to the conditions of rule 157 being fulfilled, a license to manufacture for sale any ASU drugs shall be issued in Form 25-D. |
|
Form of Loan license Rule 154 -A |
Subject to the conditions of rule 157 being fulfilled, a license to manufacture for sale any ASU drugs shall be issued in Form 25-E. |
|
Certificate of renewal of license Rule 155 |
The certificate of renewal of a license in Form 25-D must be issued in Form 26 D |
|
Certificate of renewal of Loan license Rule 155 A |
The certificate of renewal of a loan license in Form 25-E must be issued in Form 26 E |
|
Certificate of award of G.M.P. of Ayurveda, Siddha and Unani Drugs. Rule 155 B |
Must be issued to licensees who comply with the requirements of GMP of ASU drugs as laid down in Schedule T. |
|
Duration of Licence Rule 156 |
An original license in Form 25-D or renewed license in Form 26 –D, unless sooner suspended or canceled, shall be valid for three years from the date of issue or renewed. |
|
Duration of Loan Licence Rule 156-A |
An original loan license in Form 25-E or renewed license in Form 26 –E, unless sooner suspended or canceled, shall be valid for three years from the date of issue or renewed. |
|
Condition for the grant or renewal of a license in Form 25-D Rule 157 |
Conditions as specified in Schedule T |
|
Condition of license Rule 158 |
Licensee must keep proper record, shall allow the inspector, appointed under the Act, to enter the premises, must maintain the inspection book Form 35 |
|
Conditions of loan license Rule 158-A |
Licensee shall keep a proper record, must allow the inspector, appointed under the Act, to enter the premises, must maintain the inspection book Form 35 |
|
Cancellation and suspension of license Rule 159 |
After 15 days show cause to the licensee, in writing, Licensee may appeal within three months |
Conclusion
Pharmacopoeia Commission of Indian Medicine & Homoeopathy and Ayurvedic Pharmacopoeia Committee is in place to develop the quality standards and Standard Operating Procedures for Ayurvedic medicines, which are mandatory for the manufacturers to comply with. The Central and State Governments have established Drug Testing Laboratories for Ayurvedic medicines and their raw materials and 55 laboratories so far are approved or licensed in the country in accordance with the Drugs & Cosmetics Rules, 1945 for quality testing of Ayurvedic drug.
Quality certification schemes for Ayurvedic medicines are also administered as per WHO Guidelines and International Standards by Central Drug Standards Control Organization (CDSCO) and Quality Council of India (QCI) respectively.
Read our article:How one can apply for Wholesale Drug License in India?











