By creating an innovative and globally competitive industry in India, supported by best-in-class infrastructure, an enabling ecosystem, a streamlined regulatory framework, and qualified workforce, the medical devices sector will be put on an accelerated growth path to increase access and affordability of products and services of excellent quality to meet the evolving needs of patients. With increased access to patent-centric, cutting-edge, and reasonably priced healthcare items, India will be able to take the lead globally in the production of medical device products. The Drugs (Price Control) Order, 2013 (Paragraphs 6 and 19, Drugs (Price Control) Order, 2013) gives the Pricing Authority the Authority to regulate drug and medical device pricing. Using the trade margin rationalization technique (TMR approach), the Pricing Authority has, in this case, set a maximum retail price for the five medical items that were previously mentioned. In this article, we will discuss more about National Pharmaceutical Pricing Authority (NPPA).
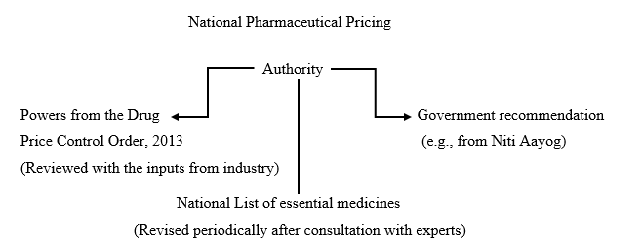
With increased access to patent-centric, cutting-edge, and reasonably priced healthcare items, India will be able to take the lead globally in the production of medical device products. The medications have given the Pricing Authority to regulate the cost of pharmaceuticals and medical devices. The Niti Aayog[1] released a consultation paper in 2017 titled “Rationalisation of trade margins in medical devices” as a result of discussions between the healthcare sector and the government in India about using trade margin data to rationalize the cost of medical devices and drugs.
About National Pharmaceutical Pricing Authority (NPPA)
On August 29, 1997, the Government of India (GOI) passed a resolution which established National Pharmaceutical Pricing Authority. It serves as an independent panel of experts for regulating the price of necessary and life-saving drugs and medical devices. The Authority has implemented the Department of Pharmaceuticals (DoP) and the Drugs (Prices Control) Orders of 2012. All medical devices listed in Schedule-I of the DPCO, 2013, are given a ceiling price by NPPA, which also keeps track of annual price increases for both scheduled and unscheduled drugs. It has so far set a cap price for 950 new drugs and 856 planned formulations. In the public interest, it has restricted the pricing of 106 anti-diabetic and cardiovascular drugs, stents, and knee implants by using the authority granted by Paragraph 19 of the DPCO of 2013.
Since its founding, it is expected to have helped consumers save close to INR 11,500 crore. Indian Pharma is currently in a unique position as the global pharmacy. Among poor nations, it is the top supplier of generic drugs, vaccines, and anti-retrovirals. The $33 billion pharmaceutical business is booming, and it has a substantial export market. Therefore, it is crucial to support Indian pharma while simultaneously ensuring fair competition for international businesses under the ‘Make in India’ initiative. In India, however, out-of-pocket medical costs continue to be the leading factor in families falling below the poverty line. Under allow its control, the Indian government included drugs under section 3 of the Essential Commodities Act of 1955. As it affects both the economic sustainability of disadvantaged groups of society and the quality of public health, the government is dedicated to enhancing access to necessary and life-saving medications. According to the DPCOs, NPPA aims to achieve a balance between the consumers’ interests and the pharmaceutical industry.
Through the Price Monitoring and Research Units (PMRUs) at State levels, the NPPA extended outside of Delhi for the first time in order to increase monitoring and public awareness. The office of the DCGI or Drug Controller General of India, State Drug Controllers, and NIPERs are actively working together to collect data, regulate the industry, and enhance services to the public. In order to make a legitimate contribution to policy formation at the Department of Pharmaceuticals and NITI Aayog, NPPA also attempts to do parallel research and studies.
As a regulator, the National Pharmaceutical Pricing Authority’s job is to foster the growth of the Indian pharmaceutical industry into a global leader while simultaneously promoting national health through the availability and affordability of pharmaceuticals and medical equipment.
Here is a diagram outlining each National Pharmaceutical Pricing Authority component for your convenience:

What are the Functions of National Pharmaceutical Pricing Authority (NPPA)?
The following are the functions and duties carried out by India’s National Pharmaceutical Pricing Authority:
- To put into effect and uphold the Drugs Price Control Order’s (DPCO) 1995/2013 provisions in line with the Authority granted to it.
- To carry out and/or support pertinent research about the cost of medications/formulations.
- To keep an eye on medicine supply, spot any shortages, and take action if necessary
- To gather and retain information on bulk pharmaceuticals and formulations on production, exports and imports, individual company market shares, company profitability, etc.
- To handle any legal issues resulting from the Authority’s judgements.
- To provide the Central Government with advice on alterations or updates to the drug policy
- To support the Central Government on legislative issues involving drug prices.
Significance of the National Pharmaceutical Pricing Authority in India
Under this section the author has explained the importance of National Pharmaceutical Pricing Authority for the country with all the major step it has taken to uplift the pharmaceutical industry during the Covid times.
- The National Pharmaceutical Pricing Authority (NPPA) makes sure that the costs of some essential medicines and medical equipment are fixed so that every person in the nation may easily afford and access them. According to the law, no supplier is allowed to sell a medication or medical equipment for more than its Maximum Retail Price (MRP).
- National Pharmaceutical Pricing Authority was extremely important during the country’s pandemic period since many medical providers were selling pharmaceuticals at excessive costs that were out of reach for many people. The Authority set the pricing; as a result, making them easily accessible to all the citizens.
What Are The Powers Given To The Authority?
You can better grasp the extensive authority granted to the National Pharmaceutical Pricing Authority by looking at the following table:
| S. No. | Power of the authority | Description of the power |
| 1 | Calculation of process | Calculating the prices of: Drug formulations (i.e., a medicine processed or containing one or more drugs);Active pharmaceutical ingredients (i.e., any pharmaceutical, chemical, biological/plant product used in a formulation). |
| 2 | Control of prices | The Pricing Authority (PA) can put a cap on the prices of drug formulations in instance of the absence of price reduction due to the absence of competition. |
| 3 | Notify DFs and APIs | Notify active pharmaceutical ingredients & drug formulations – even if they don’t feature in the Essential Medicines List in the public interest |
| 4 | Price Monitoring | The Pricing Authority has a duty to keep a check on the Maximum Retail Price short form MRP of all active pharmaceutical ingredients & drug formulations, whether/not they are listed in the Essential Medicines List. This is where the Pricing Authority derives its powers of oversight of medical devices. The Pricing Authority make sure that manufacturers & importers do not increase the MRP more than 10 per cent of the MRP of the previous year, through its price monitoring functions. Where an increase is made beyond 10 per cent, the Pricing Authority has the power to reduce it for the following year. The Pricing Authority can recover overcharged amounts |
| 5 | Monitoring availability of Listed drug formulations | The Pricing Authority has the duty to keep an eye on the availability of these scheduled drug formulations and active pharmaceutical ingredients. Manufacturers and importers these drugs are required to report the following information quarterly: Details of the manufacturer or importer, this include – name and address of the company;Details of the marketing company (if any), this include – name and address of the company;Records related to the production or import for each quarter of the year |
| 6 | Call for records and conduct inspections | It is the duty of all the manufacturers and importers to maintain records related to the sale of active pharmaceutical ingredients & drug formulations and the Pricing Authority has all the right to request these records. |
| 7 | Segments exempt from Pricing Authority oversight | In the year 2019, the Department of Pharmaceuticals amended the Drug Price Control Order of 2013 to exclude the following types of drugs from price control and monitoring system: Drugs for the treatment of orphan diseases, for example: diseases affecting less than five-lakh people in India); The order has also given five-year exemption from price control and monitoring system to manufacturers of a ‘new drug’ patented under Indian patent law. New drug is defined a drug that has not been used in India, or is being proposed for use with new dosages & indications; or a vaccine, gene therapy/novel delivery system for the drug. |
Initiatives taken by the National Pharmaceutical Pricing Authority
The National Pharmaceutical Pricing Authority is also known as NPPA. This Authority has its headquarters in New Delhi and has established Price Monitoring and Resource Units (PMRU) in all Indian States and Union Territories to expand its reach across the nation. The Authority’s most significant initiative was CAPPM (full form consumer Awareness, publicity and Price monitoring); it helped establish the PMRUs nationwide.
Recent Developments and Expanding Scope
The Health Ministry broadened the definition of a medical device in February 2020 to include software designed to diagnose, prevent, treat, monitor, or alleviate any illness, ailment, handicap, or damage. With this expanded definition, more gadgets will eventually be controlled. Additionally, it implies that the Pricing Authority will have more devices available to manage and monitor prices as more gadgets become “notified”.
In a meeting conducted in late January 200, the Pricing Authority accepted the potential of this heightened control. The Pricing Authority agreed to study the pricing details of the new gadgets that the Health Ministry had informed them about during this meeting. The sectors that produce or import four recently notified medical devices—nebulizers, blood pressure monitors, digital thermometers, and glucometers—are presently submitting information to the Pricing Authority.
This extra layer of compliance might hinder India’s Atmanirbhar Bharat initiative in the healthcare sector by disincentivizing an industry already burdened by compliance. Recently, the Commerce Minister emphasized how India has the lowest manufacturing costs for pharmaceutical goods worldwide, saying that, in an ideal world, innovation could cut pharmaceutical product costs. It is necessary to assess and harmonize all rules and regulations impacting the medical devices industry’s capacity to deliver inexpensive healthcare if the goal is to enhance access to affordable healthcare. Carve-outs have been made, even if only temporarily, for additional medicinal goods, as stated in the table. Some registered medical devices that were produced under programmes like the Production Linked Incentive Scheme may be eligible for time-limited carve-outs.
Conclusion
The most vital and fundamental component of health is medicine. To treat an illness, any system of medicine needs medications that meet the necessary standards. The daily lives of individuals now inevitably include taking medications. The scope and significance of medicine have significantly expanded with the emergence of new illnesses, particularly those like the present Covid – 19 epidemic. Access to healthcare services also involves the affordability of high-quality medical equipment.
The pharmaceutical industry is essential to India’s economic growth and population health. The pharmaceutical industry is responsible for drug invention, development, manufacture, and marketing. The government is responsible for ensuring the availability of life-saving medications at fair pricing by taking into account the interests of both the manufacturers and the consumers. The National Pharmaceutical Pricing Authority (NPPA) is the watchdog in India that regulates medicine pricing in order to protect public health. This article’s primary goal is to identify the various roles that the NPPA has played in regulating medicine pricing in India.
Read Our Article: Importance Of Medical Device Compliance In India – An Overview