It can be observed that usage of different medical devices has taken a hike, and trading of different medical devices has increased manifold due to the outbreak of pandemic COVID-19 worldwide. One of them is the ‘Infrared Thermometer,’ which is used to measure a person’s body temperature through electronic means. It has proven to be very beneficial during this unprecedented pandemic situation as it is a reliable, safer and more comfortable option for measuring body temperature.
Overview on Infrared Thermometer
People from all over the globe primarily rely on safeguarding the interest of the public at large on the data provided by such an infrared thermometer. There is a requirement to understand with respect to approvals and registrations with the stipulated laws governing such devices/instruments are in place. Those are required to manufacture, import, and/or sell such an Infrared Thermometer in the Indian marketplace. Moreover, it is mandatory to check the compliances that need to be followed.
This is quite a new schedule in today’s world with Infrared Thermometers. For that purpose, we shall be discussing in this article in brief on various approvals, registrations, and compliances that need to be done before selling an Infrared thermometer in the Indian marketplace. This article will serve you all the analysis, including various provisions of these following Acts, and will be glancing at the surfaces of various appropriate clauses.

What are the Compliances Needed under the Legal Metrology Act, 2009
There are several Compliances needed under the Legal Metrology Act, 2009[1] concerning Infrared Thermometers.
Those are as follows:-
Authorization of measuring instrument: Model
- The Infrared Thermometer is used to quantify the body temperature of a person. Moreover, it is to be noted that according to provisions of 2(w) of LM Act ‘weight and measures’ including Infrared Thermometer defined under the Act, including weighing and measuring instruments of any nature.
- In pursuance to provisions of Rules, 2011 (Model Approval Rules) & Rule 2(1) (b) of Legal Metrology (Approval of Models), every such weighing and measuring instrument is termed as ‘model’ such as Infrared Thermometers. Any person who is planning to manufacture or import any weighing instrument, including infrared thermometer under provisions of Section 22 of the LM Act, has to obtain model approval from the absolute authority.
- Every application seeking approval of the model of Infrared Thermometer shall be made to Director, Legal Metrology Act registrtion Department, in pursuance to provisions of Rule 5 of the Model Approval Rules. It should attach along with documents and samples of Infrared Thermometer with the prescribed fees and information.
- The concerned Director shall forward such application after such submission to the laboratory for its testing of Infrared Thermometers. Afterward, it shall send the report to such Director concerning elements used in such Infrared Thermometer and standards and compliance status of methodology.
- After that, a letter of approval will be issued by such Director allotting a unique Model No. of a model or measuring instrument for such an Infrared thermometer. Its manufacturer or importer shall display it on every piece of Infrared thermometer.
- Every weighing or measuring instrument employed or intended to be used in any transaction or protection in pursuance to provisions of Rule 13 (General) of Legal Metrology and Rules, 2011(LM General Rules) shall conform to the details mentioned under Seventh Schedule. It should include laterally with the constructional details, physical characteristics, performances and tolerances, configuration, materials, etc.
License Registration for Importer/Manufacturer/Dealership
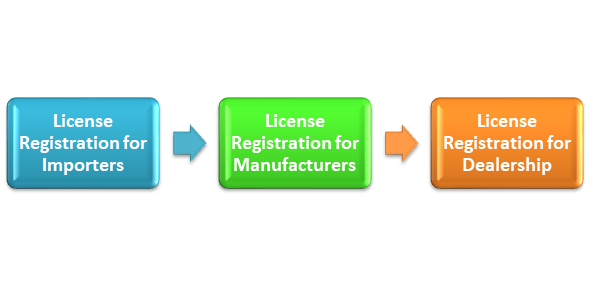

License Registration for Importers
- According to the definition of ‘import’ mentioned under Section 2(e) of the LM, Act means “bringing into India from a place not inside India” with its grammatical differences and similar expressions.
- No importer can import any such weighing and measuring instrument such as Infrared Thermometers from offshore until and unless he gets registered as importer; according to the provision under Section 19 of LM Act, read with Rule 15 of LM General Rules.
- The application shall be made to the Controller of Legal Metrology Department of the state seeking registration as Importer of Infrared Thermometers. After that, the item is going to be imported. Such a controller will forward the application with its report to the Director, Legal Metrology Department, who will commend such registration as importer.
- Moreover, it is essential to understand that this importer registration is dispersed from obtaining the Importer and Exporter Code given out by (DGFT) Director General of Foreign Trade.
License Registration for Manufacturers
- The manufacturer is the person who either manufactures any weighing or measuring instrument or manufactures such as Infrared Thermometers or any part of it, according to the definition of ‘Manufacturer’ mentioned under Section 2(i) of LM Act.
- It accumulates the rest of the parts or assembles the complete elements and claims the end product as manufactured by him, i.e., Infrared Thermometers-weighing or measuring instrument. Moreover, according to Section 23 of the LM Act, there is an explicit restriction on the manufacturers until he obtains a manufacturing license from the controller of the state in which the manufacturing is to be done to manufacture any weighing and measuring instrument counting Infrared Thermometer.
- Under Section 14 of the LM Act, bye-laws of every state need to be checked. Before moving an application for obtaining such a manufacturing license, it should be done by the controller appointed by the State Governments.
License Registration for Dealership
- A dealer is a person who carries the business of buying, selling, supplying, or distributing any weight or measure according to the definition of dealer provided under Section 2(b) of LM Act.
- Other than the dealer who exactly tends to cover the relation of the seller and end consumer without any intermediary dealer between, it also includes in its domain commission agents, manufacturers, importers who sell, supplies, and allot distributions of any weight and measures to any person.
- Moreover, no person (dealer) shall sell or possess for sale any weighing or measuring instruments under section 23 of the LM Act until he fetched a dealership license from the ‘controller of state’ in which the dealer is going to sell such ‘weighing and measuring’ instrument.
- This license will be essential to be attained before selling In the case of Infrared Thermometer also. Additionally, state bye-laws are required to have adhered since the power to grant such license is in hand of the controller, which is appointed by the state governments.
License Registration compliances for Packer and disclosure
- No person will manufacture, import, pack, sell or possess for sale any pre-packaged commodity such as Infrared Thermometers under Section 18 of the LM Act until or unless all the norms and disclosures prescribed under the instructions has been made or fulfilled with.
- According to the definition of ‘Pre-packaged commodities under Section 2(l), any product will be considered as a pre-packaged commodity that is getting packed in absence of its purchaser.
- Packer is any individual who is pre-packing any item or product in any form of package irrespective of its design in virtue of Rules, 2011 (‘Package Commodities Rules’) Rule 2(g) of Legal Metrology (Packaged Commodities). Those packing it in units are suitable for sale in the marketplace.
- Every Packer must obtain the packer registration for Infrared Thermometers by making the application to the Director or Controller according to Rule 27 of Package Commodities Rules, as the case may be along-with the prescribed documents.
- Such a submission of the pre-packing of any product/Infrared Thermometers must be made within 90 days. Also, they must make necessary disclosures and declarations, including the manufacturer, importer, Packer, date of manufacturing, expiry date, etc. as prescribed under Packaged Commodities Rules.
- Package items/commodities rules are applicable to every sort of product including Infrared Thermometer, Unlike the case of model approval, import license registration, and dealership license registration is restricted to weighing and measuring instruments, registration as Packer and disclosures.
What are the Main Verifications and Stamping Requirements must be complied with?
- Every individual who is having any ‘weighing or measuring’ as per Section 24 of the LM Act instrument has to get every single piece of such instrument confirmed and stamped from the controller. Before the apparatus (Infrared Thermometers) is sold and placed to usage the person, should abide by paying the prescribed fees.
- The power to examine such verification and stamping has been conferred in the controller of the ‘state or zone’ in which the person keeping such a weighing and measuring device is situated.
- The determination behind such verification and stamping requirement is to ensure essential disclosures have been made, and the product encounters standard quality requirements suggested under the LM Act.
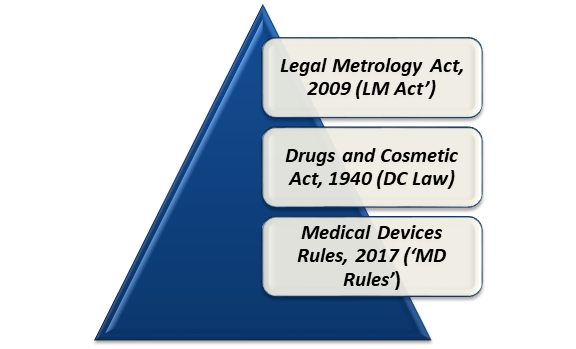
What are the Compliances Needed under Drugs and Cosmetic Act, 1940?
The Drugs and Cosmetic Act, 1940 (‘DC Law’) and Medical Devices Rules, 2017 (MD Rules), which came into effect from 01st January 2018, are known to be the laws governing compliances obligatory for importing, manufacturing, or selling of Infrared Thermometer in India.
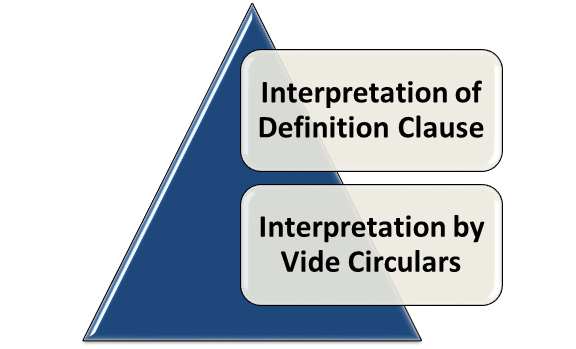

Interpretation of Definition Clause
- The definition of ‘Drug’ provided in its sub-clause (iv) under Section 3(b) of DC Law primarily includes all such devices envisioned for internal or exterior diagnosis of any disease such as Infrared Thermometers in human beings or wildlife creatures.
- Moreover, the definition of ‘Medical Devices’ refers to the instruments or devices mentioned under Rule 3(zb) clause (C)of the MD Rules, which has been notified under Section 3(b)(iv) of DC Law by the Central Government from time to time as.
Interpretation by Vide Circulars
- The ‘Digital Thermometer/Infrared Thermometers’ included under the definition of ‘Drugs’ under DC Law by virtue of the notification of Central Government dated 03rd December 2018.
- Later on, Digital Thermometers/ Infrared Thermometers got categorized into Class-B category, which basically belongs to the class of low, moderate risk items by virtue of the Drugs Controller General of India vide public notice dated 15th May 2019.
- It enables the Digital Thermometer/ Infrared Thermometers to include into the ambit of ‘Drugs’ under DC Law, which was supposed to be applicable from 01st January 2020. However, as per another notification dated 27th December 2019, such effective date now has been extended to 01st January 2021.
- One another vide notification was issued by the Central Government introducing an amendment to MD Rules by including a new chapter, namely ‘Chapter IIIA-Registration of Certain Medical Devices dated 11th February 2020′. The provision of this new chapter will be obligatory in nature after 18th Months of the commencement date of this chapter, which is 01st April 2020, and presently applicable on a voluntary basis.
- By providing the requisite information under the above chapter, the manufacturer and/or importer of any such digital thermometer/ Infrared Thermometers will register such device by means of the Central Drugs Standard Control Organisation (CDSCO)
- As a consequence of the above said, a registration number will be allocated to the manufacturer/importer, which shall be stated/displayed on the label of such medicinal device.
- The central government has introduced another circular by adding a new annexure to Schedule VIII of MD Rules, including Digital Thermometers/ Infrared Thermometers as of now and an accurate list of medical devices exempted from complying with MD Rules.
- But then again, such exemption is available only for a specific time period depending on the class in which the medical device is subsiding. Such exemption shall cease after a period of thirty months from the date of notification for Class-B concerning to a digital thermometer/ Infrared Thermometers.
- The Central Government has issued another vide notification on the same date 11.02.2020 which tends to include all devices includes the following:-

- It will include software or an accessory irrespective whether used alone or in combination intended by its manufacturer to be used for diagnosis, anticipation, monitoring, conduct, or alleviation of any disease or disorder under the DC Law into the description of ‘drug.’
What are the compliances in the Post expiration of 30 months?
Under the MD Rules and DC Law, Such a Digital Thermometer/ Infrared Thermometers will be considered as a medical device. As the case may be, all extant provisions need to be complied by manufacturer/importer.
Those are as follows-
- Apply an application for registration of Digital Thermometer to Central Licencing Authority immediately; the action will be required through the identified online portal by CDSCO.
- For the manufacturer of Digital Thermometer/ Infrared Thermometers, which is falling in Class B according to provisions stipulated under Rule 20 of MD Rules, such manufacturer shall make an application to the State licensing authority for procuring manufacturing license or loan license in Form MD-3 and 4 respectively. It should be done along with the prescribed fees and with necessary documents or information declared therein.
- Point to be noted that such a manufacturing license or loan license is obligatory to be obtained on an earlier basis. Importer of Digital Thermometers / Infrared Thermometers is required to obtain an importer license according to Rule 34 of MD Rules. Moreover, an application will be needed to be made in Form MD-14 to the Central Licensing Authority for the same purpose.
Conclusion
Assuming the circumstances as mentioned above, there are a lot of compliances to be done and approvals or registrations obligatory to be obtained before dealing in and industrializing Infrared Thermometers in India. It must abide by all the applicable Central and State laws which are required to be fulfilled. However, the examination report says that on present-day, only LM Act is the correct incomplete manner, and there are some reductions provided under DC Laws for some dated of the period.
Point to be noted that the requirement of prior approvals and registrations stipulated under LM Act has been relaxed to some extent. It was done in consideration the critical requirement of such Infrared Thermometers throughout this COVID-19 pandemic outburst in the country and bearing in mind the issue regarding time taking the process of model endorsement.
It is necessary to be diligent while dealing with it as any negligence may bring huge penalties or unnecessary costs since various Central and State laws are applicable in the case of Infrared Thermometers. The practice has been followed to obtain an undertaking from the importer with the department that they will comply with such requirements by taking post-approval within three months.
Our CorpBiz group will be at your disposal if you want expert advice on any aspect Infrared Thermometers and its Registrations & Permissions. We will help you to ensure complete compliances concerning all the requirements based as per your desired activities, ensuring the fruitful and well-timed completion of your work.
Read our article: Import License of Drugs and Cosmetics in India