The healthcare industry has confronted proactive changes over the past few years, the increasing significance of contract manufacturing organizations and third party pharma manufacturing business, swift adoption of cutting-edge technologies such as AI and automation, & increasing pressure from government across the globe to minimize the healthcare burden were some of the themes that influenced the pharma sector in the recent past. The most prevalent issues of the industry have been confronting are the massive R&D spend & ever-decreasing returns on investments. Pair it with pressure from governing agencies on drug pricing and the need to improve efficacy & productivity across the value chain. Outsourcing has remained the prominent choice for pharma companies for years because it will help them to improve operational efficacies, expand geographical presence, & escalate therapeutic expertise & on-demand services. This blog underpins the detailed blueprint on how to set up a profitable third party pharma manufacturing business in India.
Outsourcing or contract-based manufacturing is prominently utilized in the Pharma sector because it helps companies to;
- Meet consistent demand
- Offers competitive pricing
- Increase production volume
- Offer better resistance to surging competition
Since the concept of third party pharma manufacturing business is thriving rapidly, startups with moderate funding can jump into this venture can earn sustainable profits for years to come.
What is the growth potential of third party pharma manufacturing business in India?
Owing to the Cornovirus pandemic, the pharma sector sought third party pharma manufacturing in a big way. Its growth from 934.8 million dollars in FY 2017 to 1.17 trillion dollar gives an insight into the exponential boom this sector is witnessing. However, the real stats could be much greater owing to better healthcare becoming a priority in 2020.
To keep up with the surging demand, the pharmaceutical companies have been burdened with greater financial performance, particularly when it comes to buying & running pricer equipment for the mass production of drugs. To overcome this, many entities have started to opt for third party pharma manufacturing firms that have the equipment and relevant resources to carry out a cost-effective production. Contract manufacturing is turning out to be a game-changing strategy for pharma companies facing massive demand.
India has emerged as a prominent player in the Active Pharmaceutical Ingredients, Key Intermediates & Formulations. Majority of the nations consider India as one of the prominent sourcing based as India has huge number of USFDA certified facility outside the US.
Overseas and Indian pharma companies confront massive challenges related to ever-changing regulations in the outsourcing process. Therefore to overcome such obstacle, it becomes vital for the pharma companies to secure a reliable pool of experts. Most mid-sized pharma companies have approved manufacturing units, skilled workforce, & good technical know-how, etc. The international companies, on the other hand, encounter issues in identifying dependable supplier/manufacturer.
Legalities for establishing third party pharma manufacturing business
Following are the list of legalities to function as a viable third party pharma manufacturing business in India.
Opt for a Relevant Company Name
Selecting a company name for a new establishment is always a tricky affair and most vital task. In the marketing landscape, we acknowledged a product through its name. When we see a company or brand logo, a name strikes our mind. Before opting for a company name, take the following facts into account.
- The company name should perfectly blend with the nature of your operation. Since you intend to deal with pharma products, your company name should reflect the same. Choose a name that ends with terms like cure, pharma, bio, life sciences, healthcare, so on and so forth.
- The company name should adhere to the uniqueness, and it must not conflict with the name of other entities. This will help you keep the IPR dispute out of the equation.
- The company name should be short and easy to spell
- Avoid affixing silent characters in the company name as they have a tendency to create confusion in the customer’s mind.
- The name should be purely technical.
Secure Company Registration
One can register a pharma-based company in various ways. Presently, the following business structures are available to entities intending to function in a legalized way:
- One Person Company (OPC)
- Limited Liability Company (LLP)
- Private Limited company
- Public Limited Company
- Limited Liability Company (LLP) comes under the ambit of LLP Act, 2008; meanwhile, the rest of the company structures are regulated by Company Act, 2013.
- Company incorporation under LLP is done on the basis of an LLP agreement that acts as a written contract between the partners.
- After successfully drafting the said agreement, the applicant needs to submit the same before the registrar of the respective state.
- Ministry of corporate affairs facilitates the registration of company structures like a private limited company, OPC, and Public limited company. The entities seeking incorporation under these structures need to file an e-form SPICe+ on the MCA’s portal[1].
Here is the list of documents that one needs to arrange before engaging with the incorporation process for the above business structures:
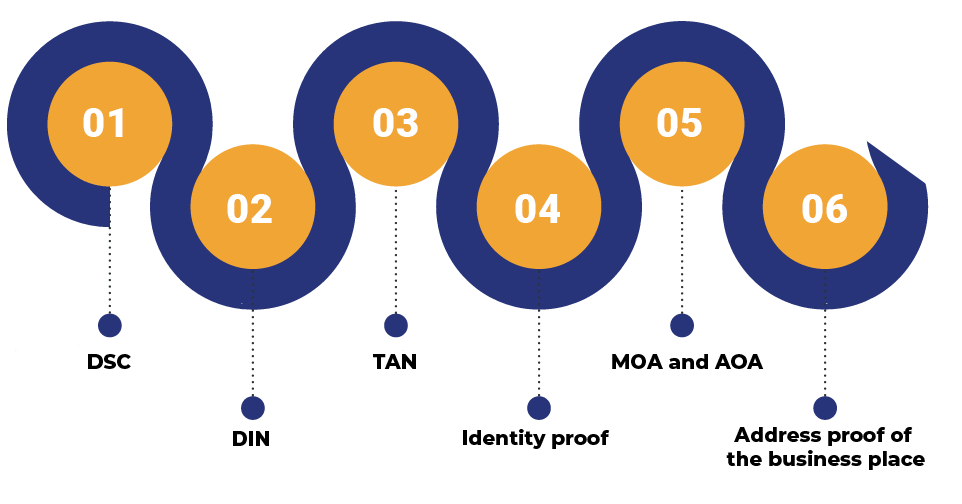

Secure the Wholesale Drug License
The licensing authority functioning under the aegis of the State government facilitates the wholesale drug license by way of appointed individuals acting as drug inspectors or senor drug inspectors. To avail of such a license, you need to submit a prescribed application before the licensing authority of the respective state accompanied by the following documents:
- Pharmacist registration certificate
- Appointment letter of the competent individual
- Copy of address proof such as rent agreement and ownership agreement
- Xerox of the Refrigerator purchase bill
- Copy of academic qualification of the pharmacist
- Copy of DOB certificate
- Partnership deed (in case of partnership firm)
- Copy of Incorporation certificate, list of directors, MOA, and AOA, etc
- Affidavit of Directors/partners/proprietor
- Detailed schematic of the manufacturing unit
Steps to be taken after securing the above documents
- Head over to the official portal of the state licensing authority. Note: In some states, the online filing is not available, and thus applicant needs to opt for a manual approach for obtaining the license)
- After filing a prescribed e-form for the license, upload the aforesaid document in the given format.
- After successful filing, the portal will generate an acknowledgement receipt.
Step 4: Avail GSTIN Registration
Goods and Service Tax (aka GST) refers to indirect taxation, which subsumed all prevailing indirect taxes imposed by the State & Central Government. The tax rates under GST are imposed in accordance with the GST Act 2017.
GST encompasses the following taxpayers under its ambit:
- Having an annual turnover more than Rs 40 lacs (In some states, this limit has been slashed down Rs 20 lacs)
- Engaged with the inter-state supplies of goods and services
Apart from that, GST covers the following taxpayers as well:
- Casual taxable individual/ ISD
- Non-resident taxable individual
- Casual taxable person / Input Service Distributor (ISD)
- Non-resident taxable person
- Supplier of goods via an online portal
- Any service provider
- Liable to address tax obligations under the reverse charge mechanism (aka RCM)
- TDS/TCS deductor
- Online data access or retrieval service provider
Facts to Keep in Mind for Staying Competitive and Profitable
- Pricing is critical and probably the next key thing after your brand value which will keep your business running. Offering competitive pricing can be challenging if there are too many competitors in the market. To overcome this trouble, you need to set your charges accordingly by evaluating the average price offers by all competitors—the idea is to offer a price that doesn’t sound impractical from the client’s standpoint. Also, you need to focus on a specific drug category that can ensure a better profit margin.
- Many third party manufacturers are incorporating AI and other cutting-edge technology solutions to become more cost-effective
- & improve production time.
Conclusion
Third-party pharma manufacturing is a profitable business model that revolves around stringent operation-based compliances. Therefore it is essential for startups to do their homework properly before venturing into this business landscape. If case you confront any difficulties on the licensing part, feel free to avail advise from CorpBiz’s associates.
Read our article:Cold Storage Facility: Legalities, Scope, and Benefits










