Drugs are playing an important role in assuring the good health of citizens of any country. Drugs are different from other merchandise, and because of that, the Government has laid down inflexible laws. The regulation governing the manufacture & sale of drugs is provided in the Drugs and Cosmetics Act, 1940 and Rules framed under it.
The rules made under the Drugs & Cosmetic Rules, 1945 exercise control over drugs at the raw material stage during manufacture, sale, and distribution. It is up to the time it is supplied to a patient or consumer by a Pharmacist in a retail Pharmacy, Hospital or Dispensary.
The Principles Related To Drug License Registration under Drugs & Cosmetic Act, 1940
The Drug and Cosmetic Act regulates the manufacture, sale, distribution, and import of drugs since 1940. The drug license allows doing business activities related to pharmaceutical products. Drug license depends on certain terms of conditions provided under the Act that every druggist needs to follow for the retention of a drug license. The Government main objective was to protect the public interest by imposing such conditions under the Act.
Provisions Related to Retention of Drug License in India
When you are planning for retention of drug license, then you should check the below-mentioned criteria that you are fulfilling the conditions of Drugs & Cosmetic Act, 1940[1] or not;
- An applicant for retention of drug license must have at least four years of practical experience in drug distribution.
- A drug licensee can only manufacture a drug under the supervision of an experienced pharmacist.
- It is forbidden by the law to stock or sells any drug after its expiry.
- A drug retailer cannot provide any drug to a minor or a child below 18 years.
- The drug retailers should make entry in the register each time they sell any drug to maintain the record for future reference.
- The medicaments to cure animals must be labeled as “Not for human use, for treating animals only.”
- Drugs that fall under Schedule H or Schedule X category or have a validity of period of 2 years. Thus, such drugs should only be provided to registered medical stores like dispensaries, nursing homes, hospitals, and medical practitioners. Moreover, it is essential to sign a written order to provide these drugs.
- A drug licensee cannot preserve any drugs at his place intended for the free sample distribution to a certified physician.
- If a medical practitioner prescribes any medicine, it should be under the guidance of a Regd. Pharmacist before its supply or retailing.
- If a pharmacist has stored an expired medicine to claim a rebate from the Income Tax Department, it shall not be regarded as an offense unless the drug is not indented for sale purposes.
Amendments under Drugs & Cosmetic Act, 1940

The primary objective to amend the Act is to provide the safeguard to licensee in terms of retention of drug license.
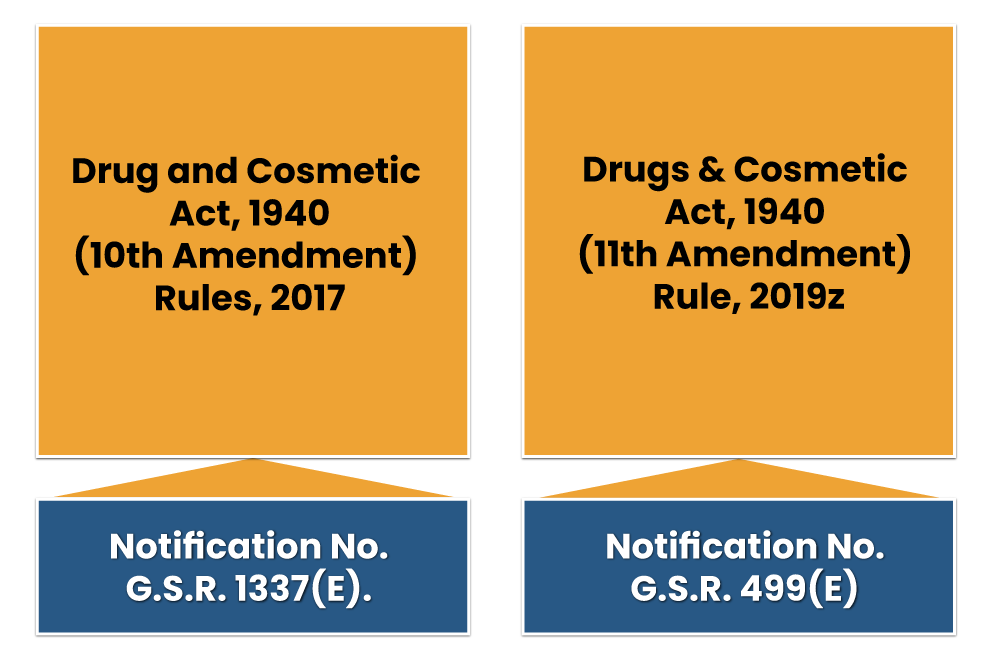
Drug and Cosmetic Act, 1940 (10th Amendment) Rules, 2017
On dated 27th October 2017, the authority of Drugs and Cosmetics has announced the Drugs and Cosmetics (10th Amendment) Rules 2017 through notification no. G.S.R. 1337(E).
- As per the amendment, the sale and manufacturing of the drug license shall have validity with no time restriction. The licensee shall have to pay the fee for the retention of drug licenses every five years.
- The license shall not remain valid forever provided that the licensing authority suspends or cancels it on certain grounds.
- Further, it stated that the Central and State Drug inspectors should inspect the place at least once in three for manufacturing drug licenses.
- The Government has also modernized the sale licenses and renewal of manufacturing by easing the provisions. Thereby it assist the licensees to continue their business for the long time.
Drugs & Cosmetic Act, 1940 (11th Amendment) Rule, 2019
The Ministry of Health and Family Welfare on dated July 17, 2019, in consultation with the Drugs Technical Advisory Board has announced the Drugs and Cosmetics (Eleventh Amendment) Rules, 2019 through notification no. G.S.R. 499(E) to further amend the Drugs and Cosmetics Rules, 1945. The Notification shall come into force by the publication in the Official Gazette.
The changes made in the Drugs and Cosmetics Rules, 1945 are as follows:-
- A new Rules 77 and 83 has been inserted specifying duration of license and loan license where a license issued in Form 28, Form 28B and Form 28D shall remain valid. It is valid if the licensee deposits a drug license retention fee before the expiry of period of every succeeding five years from the date of its issue, unless it is suspended or cancelled by the licensing authority.
- The FORM 33 has been omitted and a new Form 32A has been substituted.
- The Rule 150J which talks about renewal of certificates has been omitted.
- Amendments are made in Schedule A, in Form 24C, Form 26J and Form 37.
Effect of Amendments for Retention of Drug License
- Renewal was a time consuming process. It will prevent time consumption after the announcement of new guidelines.
- New guidelines will create a transparency in retention of drug license process.
- It will decrease the work overload of drug department.
- After the announcement of new guidelines the inspection will be compulsory. Due to this the licensee will be liable to fulfill all requirements as drug department may conduct inspection on regular basis.
- This amendment is made in order to implement good manufacturing standards
- After the releasing of notification the quality of products manufactured in India may improve as manufacturing standard compliance could be regularly checked.
- More control of Drug Authorities will be at ground level for maintaining standards and availability of quality products.
- Now Drug Department can concentrate at proper implementation of laws and rules according to Drug and Cosmetic Act.
Conclusion
To make the process of doing great business in the pharmaceutical industry in the easier way, the Central Drugs Standard Control Organization (CDSCO) has announced to make it mandatory for pharmaceutical companies and chemists to periodically pay a license retention fee instead of the current ambiguous practice of renewal of licenses. The recent amendments under Drug and Cosmetic Act, 194o for retention of drug license will bring a positive change. Retention of drug license will be beneficial for both the Drug Department and drug license holder. Kindly contact with Corpbiz experts to know more about retention of drug license.
Read our article: Legal Obligation under CDSCO for Obtaining Wholesale Drug License













