The Indian Pharmaceutical Sector is one of the significant growth stimuli for the Indian Economy. The pharmaceuticals industry is the 3rd largest in terms of volume & 13th largest in terms of value. It has now become the center stage for the manufacturing & research hub.
The Indian Pharmaceutical Market is ruled by generic drugs comprising nearly 70% of the market, whereas the patented and generic drugs make up 9% & 21%, respectively. Manufacturing drug in India is cheaper as compared to other nations such as Europe and the USA. In this write-up, we will look into the detailed procedure on how to export medicine worldwide from India.
Overview of Pharmaceutical Exports in Recent Years
- Pharmaceuticals export from India was accounted for US$ 20.70 billion in 2019-20.
- The pharmaceutical sector witnessed a growth of 12.43% to USD 17.57 billion in terms of export during April-December 2020-21.
- Indian is one the prominent supplier of generic globally (20 to 22% of the global export volume.
- The drug export during April 2020 to November 2020 was accounted for US$ 15.87 billion. Meanwhile, for the month of November 2020, it was stood at US$ 1.99 billion.
- Indian export figure for the Bulk Drugs & Drug intermediates in FY 20 stood at US$ 3.89 billion.
Procedure to be Followed to Export Medicine from India
If you have a registered drug company in India, there is a simple process to export medicine from India.
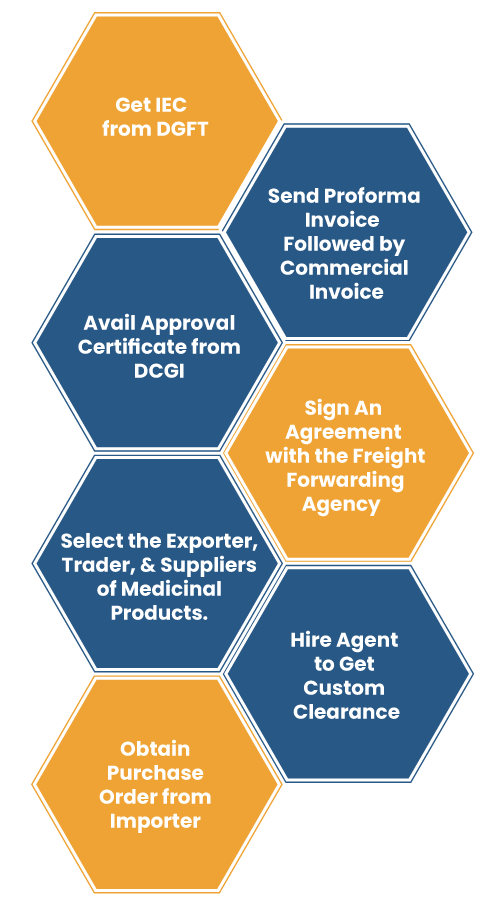

- The first step is to avail the IEC (Import Export Code*) number from DGFT.
- Locate the prospective buyers in other nations who are keen to import medicine from India.
- Register your product in the importing nation.
- Get the approval certificate from DCGI (Drug Controller General of India) to export medicine to other nations.
- Choose the appropriate shipping method or select the exporter, trader, and suppliers of medicinal products.
- Obtain the Purchase order* from the importer and send a proforma invoice* with detail like rate of drug, type of packing, freight details, etc.
- Prepare commercial invoice against the Purchase Order.
- Sign an agreement with the freight forwarding agency to dispatch the shipment to the respective nation.
- After completing the document work get the customs clearance. For this, appoint an agent who can clear the shipment by fulfilling all the custom’s requirements.
- After obtaining clearance, the shipment will be routed to the importing nation.
- Customs clearance is also necessary for the importing country
What is Import Export Code*?
Import Export Code (IEC) is a 10-digit number that works as an identification code for the individual engaged with the foreign trade. It is issued by the Director General of Foreign Trade (aka DGFT), Department of Commerce, and Government of India.
IEC registration is mandatory for conducting cross-border transactions of goods. In the absence of the Import Export Code, nobody has the right to export or import the shipment of goods.
What is the Proforma Invoice*?
Proforma invoice refers to a document that encloses information regarding the goods and/or services yet to be delivered to the importer/buyer. It also manifests estimated prices of the product in the shipment.
What is the Purchase Order*?
A purchase order, aka PO, is a document issued by the importer committing to pay the seller for the sale of products or services to be dispatched in the future. Each Purchase order has a unique number that helps both importer and seller track delivery and payment.
How to Apply For IEC to Export Medicines from India?
Procedure for obtaining IEC Number from DGFT to export medicine from India
- Visit the official portal of the Directorate General of Foreign Trade (DGFT).
- Explore the ‘Online Application’ option on the home page and select ‘IEC’ via the drop-down menu.
- Next, select the ‘Online IEC Application.’
- Provide your PAN detail for verification so that you can log in.
- After login, select ‘New IEC Application details.’
- A form will be prompted on the screen. Fill in all the relevant information in the form and select the option ‘Upload Documents.’
- Next, upload the requested documents.
- After this, select the ‘Branch’ option to add any branch details.
- Next, opt for the ‘Director’ option to add the director’s details.
- Select ‘EFT’ for the online fee submission.
- Fill in the required amount and select the bank to make the transaction.
- A draft invoice shall prompt on your screen. Make sure all details that you furnished before are legit.
- Proceed to the “Pay now” option for making an online payment.
- Complete the payment formalities via your internet banking details.
List of Particulars Required to Avail IEC
- PAN Number
- Bankers Certificate
- Current Bank Account
- IEC Code Number Application Fee
The hard copy of the application and requested documents must reach DGFT office within 15 days of its online submission.
Conclusion
Shipping medicinal products outside India is subjected to plenty of legal implications that one must fulfill without exception. After availing of the permissions above and licenses from the respective authority, you will become eligible to export medicine to overseas countries. This way you can expand your business footprint and access the new market.
One of the biggest obstacles in exporting medicine is obtaining an approval certificate from the DCGI (Drug Controller General of India[1]). One has to fulfill a long list of requirements for availing such a certificate. In case if you find it hard to do so, let our professional help you out.
Read our article: How Import License and IEC Certification Differs From Each Others?













