In India, import, manufacturing, sale, and distribution of drug is regulated under Drugs and Cosmetics Act 1940 and Drugs and Cosmetic Rules 1945. Import license is provided for the import of drugs and cosmetics subject to those which are specified in Rule 10 and Rule 10A. In lieu to obtain Grant of Import License, an application for an import license is made in the form and manner prescribed in Rule24. The licensing authority on being satisfied will grant import license. When the conditions of the license will be fulfilled, the issue of an import license will be in Form 10 or Form 10-A. The Import license unless, it is suspended or canceled, must remain valid for a period of three years from the date of its issue.
Application of Import License
An application is made in Form 8 and Form 9 respectively, for obtaining an import license in Form 10. All applications should be made through the CDSCO portal. Rule 24 of the Drugs and Cosmetics Rules deals with the procedural requirements for obtaining an import license.
Procedural Requirements are as follows:-
- The application made either by the manufacturer himself having a valid wholesale drug License for sale or distribution of drugs under the Drugs and Cosmetics Rules 1945.
- By the manufacturer’s agent (Importer) in India either having a valid License under the Drugs and Cosmetics Rules to manufacture for sale of a drug or having a valid wholesale license for sale or distribution of drugs under these Rules.
- In a power of attorney, the agreement by the manufacturer to his Indian agent has to be documented and verified either in India before a First Class Magistrate or in the country of origin.
- The application for an import license has to be followed by a copy of the Registration Certificate issued in Form 42 issued under Rule 27A.
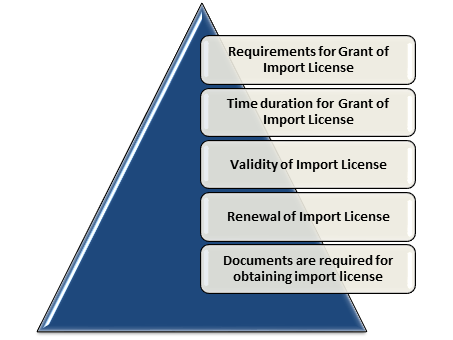

Requirements for Grant of Import License
As per Rule 25A of the Drugs and Cosmetics Rules 1945, the following conditions must be fulfilled by the applicant
- Imported substances will be sorted properly for maintaining the properties of the Drugs applies for import license.
- Where any such charge in the constitution of the manufacturer site or address takes place, the current Registration Certificate is considered to be valid for a maximum period of 3 months from the date. In the meantime a fresh Registration certificate will be issued form the licensing authority with the charged details.
Time duration for Grant of Import License
- If the application is made properly, the licensing authority will issue an import license in Form 10, within three months from the date of receipt of an application.
Validity of Import License
- According to Drugs and Cosmetics Rules, Import license must remain valid for the period of three years, until the Registration Certificate is valid.
Renewal of Import License
- Applications of renewal of import license should be submitted along with the documents required for renewal within three months of the expiry of the import license.
Documents are required for obtaining import license
- Cover Letter
- Form-8/8A duly Signed & Stamped by applicant
- Form 9 (Undertaking given by Manufacturer or on behalf of manufacturer)
- Notarized copy of Wholesale or Manufacturing drug Licence
- Copy of valid Registration Certificate (RC) on Form 41 duly attested by the Indian Agent or Importer
- Copy of permission under Rule 122A (Application for permission to import new drug) in case of New Drug in the name of the importer
Prohibition on Import of Drugs or Cosmetics
The Drugs and Cosmetics Act 1940 under Section 10 [1] have made restriction on the imports of the following:
- Drugs or cosmetics of substandard quality
- Any adulterated or spurious drug
- Any misbranded or spurious cosmetics
- Any drug which required to cure or mitigate any disease
- Any patent or any proprietary medicine which has no description of the true formula or list of active ingredients included in it
- Any cosmetics that include an ingredient that may be unsafe or harmful for consumption
- Drugs or cosmetics that are prohibited for imports under these provisions
Central’s Governments Power of Prohibition
The Central Government, after consultation with the Board, can permit the import of any drug or class of drugs not being of standard quality by making an official notification in the Gazette. This permission would be given subject to any conditions as may be required.
The Central Government can prohibit the import of a drug or cosmetic if the government is satisfied that:
- The use of such drug or cosmetic involves risk to human being or any animal.
- The drug that lacks its therapeutic value.
- The drug or cosmetics includes ingredients in such quantity for which there is no therapeutic justification.
Import of Drugs in India
The Central Government exercises regulatory control over these drugs and cosmetics imported into country through (CDSCO) Central Drugs Standard Control Organisation headed by the (DCG) Drugs Controller General of India.
- The manufacture, sale, and distribution of drugs are primarily regulated by the State Drug Control Authorities appointed by the State Government.
- The objective of the drug regulatory system in the country is to ensure availability of safe, effective, and quality drugs, cosmetics, and medical devices based on scientific excellence and best possible regulatory practices.
- Drug is defined in Section 3 of the Drugs and Cosmetics Act 1940. The Central Government has the power to declare any drugs, cosmetics, or medical devices as useful Drugs by giving notification in the official gazette.
- By virtue of the said power the Central Government has Notified Disposable Hypodermic Syringe, Disposable Hypodermic Needle, and Orthopedic Implant, Catheter, as drugs in 1989.
There are three types of import that are:
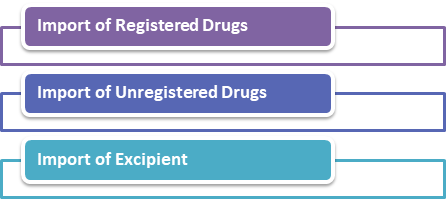

Import of the Registered Drugs
- When any drug registered in India a Certificate of Registration in the prescribed Form 41 is issued by the appropriate authority of the Central Government. When any person wants to import the registered drug, it is required to have import licenses by the appropriate authorities of the Central Government.
- As per Rules 24 and 27 of the Drugs and Cosmetics Rules 1945, the import license to import drugs that are not specified in Schedule X to these Rules will be issued in the prescribed Form 10.
- It will also apply to import of drugs which are specified in Schedule X to the Drugs and Cosmetics Rules, 1945. The import license will be issued in the prescribed Form 10A.
- In India the drugs which are specified in Schedule X to the Drugs and Cosmetics Rules cannot be purchased over the counter without the prescription of a qualified doctor. Not only that the retailer also has to preserve the prescription for a period of two years.
Read our article:Take – Off Your Business to International Market with Import and Export Code (IEC); Know IEC Code’s Benefits
Labeling on the Imported Consignment
- On every import consignment of the registered drugs a label should be affixed showing the name and address of the manufacturer, date of manufacturing, batch number, date of expiry of the drug, name and address of the importer, import license number and date. (Form 10 or 10A)
Testing on Imported Drugs
- As a safeguard, the Drug Controller office is at the Nominated Port where the import consignment arrives draws sample from the imported drug for testing to verify. It is checked that the drug which is being imported in India as a registered drug is the same drug that is actually registered in India or not.
- The samples are sent for testing at the Central Drug Testing Laboratory of the Government of India. If the result of the testing comes to the satisfaction of the Drug Controller office the import consignment is given to the importer.
- Import of any drug for the purpose of examination, test, or analysis in India is allowed subject to the import should be made against Test License issued by the appropriate authorities in the prescribed Form 11.
Import of the Unregistered Drugs
- Unregistered drug means the drug which is not registered in India hence, no import license is issued consequently. The import of unregistered drug in India is not possible. However, there are various Drug Manufacturers Associations which have granted exemption from registration requirement under the Drugs and Cosmetics Act.
- The import of unregistered drug made under Advance Authorisation (Advance License). The Government of India Ministry of Commerce and Industries has by Policy Circulars made a provision that no registration is required if the unregistered drug imported under the Advance Authorisation.
Those are subjected to the following Conditions:
- The Advance Authorisation has to be issued against valid export orders and to the extent raw material (Imported Drug) required to manufacture the final product which has to be exported.
- The final product which is to be exported must be manufactured from the unregistered drug which has been imported under the advance authorization.
- The export has to be made within 12 months from the date of the First import made under the advance authorization.
- The export of the final products made out of the drug so imported under the authorization to the same buyer against whose export order the advance authorization is issued.
- If the raw material is not possible to be utilized for manufacturing the final products which are to be exported the imported material can be used in any other products which are to be exported.
- If it is not possible to manufacture the final product from the imported materials the imported material should be re-exported.
- If re-export of imported material is not possible the same should be destroyed in presence of Jurisdictional Excise Authority.
- The advance authorization holder is required to submit documentary evidence regarding such destruction of the imported raw material or the final product manufactured from the imported material.
- The advance authorization holder has to pay applicable customs duty on the imported material against such Export Obligation is not fulfilled along with applicable interest.
For availability of safe, effective, and quality drugs, medical devices based on the scientific excellence and best possible regulatory practices the imported material under the advance authorization which is unregistered in India is not allowed to be diverted for the domestic consumption within India.
Import of Excipient
- Import of any drug some substance is used for coloring or as preservative or as filler or diluter. The substance is not active in the drug in which it is used but works as vehicle or medium for the drug or other active substances. This substance which is so used it is called excipient.
- Excipient can be imported in India without any import license issued under the Drug and Cosmetics Act 1940. However, no objection certificate (NOC) issued by the Drug Controller Office in India is required for import of such excipient. A copy of the NOC is sent to the Drug Controller Office at the port where the imported cargo is to have arrived.
- No testing is required of the material imported as an excipient. The NOC issuing authority will mention in the NOC name and address of the manufacturer, name and quantity of the item to be imported and an instruction that Not for Medicinal use.
Offenses
The following penal provisions are applicable on non-compliance with the provision:
- The import of any adulterated drugs or any spurious cosmetics which involves the risk to human life has an imprisonment for a term which may extend up to three years or a fine, which can be maximum up to Rs. 5,000 or both. On the subsequent conviction, imprisonment can extend up to 5 years.
- The import of any drug or cosmetics that are prohibited under any section of the act has an imprisonment for a term which may extend up to six months, which can maximum up to Rs 5000 or both. On the subsequent conviction, imprisonment can extend up to 1 year.
- The import of drugs or cosmetics against the provisions given in any notification issued under section 10A will be liable for imprisonment, which may extend up to 3 years, in addition to a fine which can maximum up to Rs. 5,000 or both.
Conclusion
Import License of the Schedule-X drugs (Narcotic & Psychotropic drugs) requires some conditions to be fulfilled which are Licensee must have adequate storage facility and applicant must be reputable in the occupation. The license granted must not be suspended or canceled unless the licensee has not been convicted of any offense under the Drugs and Cosmetics Act or Narcotic and Psychotropic Substances Act.
Our Corpbiz group will be at your disposal if you want expert advice on Import License of Drugs and Cosmetics in India. We will help you to ensure complete process as per your anticipated activities, ensuring the successful and well-timed completion of your work.
Read our article:Advantages of Obtaining IEC (Import Export Code) Registration in India